Translate this page into:
An epidemiological and clinical analysis of cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia
2 Department of Paediatrics, Monash University Sunway Campus, Clinical School Johor Bahru, Johor, Malaysia
Correspondence Address:
Siew-Eng Choon
Department of Dermatology, Hospital Sultanah Aminah Johor Bahru 80100, Johor
Malaysia
| How to cite this article: Choon SE, Lai NM. An epidemiological and clinical analysis of cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia. Indian J Dermatol Venereol Leprol 2012;78:734-739 |
Abstract
Background: The prevalence, clinical patterns, and causative drugs of cutaneous adverse drug reactions (cADR) vary among the different populations previously studied. Aim: To determine the prevalence, the clinical patterns of drug eruptions, and the common drugs implicated, particularly in severe cADR such as Stevens-Johnson Syndrome/Toxic epidermal necrolysis (SJS/TEN) and drug rash with eosinophilia and systemic symptoms (DRESS) in our population. Methods: We analyzed the database established for all cADR seen by the department of Dermatology from January 2001 till December 2010. Results: A total of 362 cADR were seen among 42 170 new clinic attendees, yielding an incidence rate of 0.86%. The most common reaction pattern seen was maculopapular eruption (153 cases) followed by SJS/TEN (110 cases) and DRESS (34 cases). Antibiotics was the most commonly implicated drug group (146 cases) followed by anticonvulsants (81 cases) and antigout drugs (50 cases). The most frequently implicated drug was allopurinol (50 cases). Carbamazepine, allopurinol, and cotrimoxazole were the three main causative drugs of SJS/TEN accounting for 21.8%, 20.9%, and 12.7%, respectively, of the 110 cases seen, whereas DRESS was mainly caused by allopurinol (15 cases). Mortality rates for TEN, SJS, and DRESS were 28.6%, 2.2%, and 5.9%, respectively Conclusions: The low rate of cADR with a high proportion of severe reactions observed in this study was probably due to referral bias. Otherwise, the reaction patterns and drugs causing cADR in our population were similar to those seen in other countries. Carbamazepine, allopurinol, and cotrimoxazole were the three main causative drugs of SJS/TEN in our population.Introduction
Cutaneous adverse drug reactions (cADR) are common, comprising 10-30% of all reported adverse drug reactions [1],[2] and its incidence in hospitalized patients is estimated to be 2-3%. [3] The prevalence, patterns of cADR and their causative drugs vary greatly among the different populations previously studied in Europe, Israel, and Asia. [2],[3],[4],[5],[6],[7],[8],[9] Although the majority of cADR are mild and self-limiting, severe cADR (SCAR) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) are associated with significant morbidity and mortality. [10]
The objective of this study is to (i) document the rate of cADR in our population, (ii) identify the clinical pattern of drug eruptions and the common drugs implicated, particularly in SCAR such as SJS/TEN and DRESS, and (iii) determine the outcome of patients with SCAR.
Setting
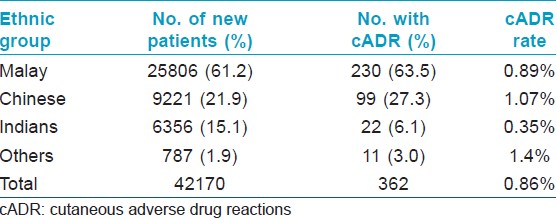
The department of dermatology in Hospital Sultanah Aminah is a tertiary referral centre for Johor Bahru district in the state of Johor, Malaysia which has a catchment population of approximately 1.8 million. A total of 203,231 visit records were documented during the study period 2001-2010 with a mean annual workload of 20,323 patient encounters (range: 16,187 to 23,044), of which 20.7% were new cases (total: 42,170, mean: 4217, range: 3403-4824). The racial composition of our new patients were 61.2% Malay, 21.9% Chinese, 15.1% Indian, and 1.9% others with a male to female ratio of 1.02:1. The majority of our new patients were between 20 to 59 year-old (53.0%) with 21.0% aged below 10 year old, 14.3% were between 10 and 19 yea -old and 11.9% were 60 year old and above. The demographic characteristics of our new patients are reflective of the 1.8 million population in Johor Bahru district.
Methods
The department of Dermatology keeps a registry of all patients with cADR since 2001 and uses mortality rate of SJS/TEN, set at less than 25%, as a yearly measure of the quality of care for patients with SCAR. All suspected cADR referred to our department are evaluated by a dermatologist and only cases with a causality grading of "probable" are registered and reported to our National Drug Monitoring Centre which is the 34 th member of the WHO (World Health Organization) International Drug Monitoring Program. We used criteria developed by the WHO to define causality. A "probable" causal association is considered to be one where there is a reasonable time sequence, the event is unlikely to be attributable to concurrent disease or other medicines and a clinically reasonable response is observed on dechallenge. Rechallenge information is not essential to fulfill this definition. [11] SCARs in this study included only DRESS and SJS/TEN spectrum of cADR.
This study was done by analyzing the database established for this cADR registry from January 2001 till December 2010. Information in this database was gathered from patients′ clinical records and our national adverse drug reaction reporting forms which were either filled in or validated by a dermatologist.
Statistical analysis
Descriptive analysis was presented. We calculated cADR rate using the reported cases of cADR as numerator and the total number of new patients seen in the department as denominator. Direct comparisons of the cADR rates between different races and age groups were performed using an online two-way contingency table calculator (http://statpages.org/ctab2x2.html). The significance level was set at P<0.05.
Results
Demographic characteristics
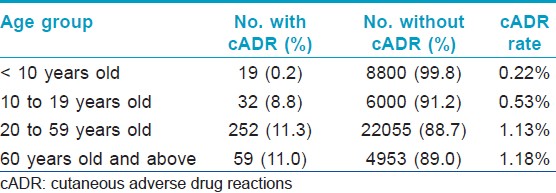
A total of 362 cADR were seen among the 42,170 new patients, yielding an incidence rate of 0.86% (yearly cADR rate range: 0.55-1.28%). Indians had lower cADR rates compared to Malay and Chinese (P < 0.0001 on both comparisons) [Table - 1]. There is no significant difference between the cADR rates of Malay and Chinese (P =0.12). The male to female ratio was 1.14:1. The majority of affected patients (67.1%) were between 20 and 59 year-old with a mean age of 39.6 years (range: 1 to 98). [Table - 2] shows the rates of cADR in patients classified under four age groups.
Clinical reaction patterns and drugs implicated
The most common reaction pattern seen was maculopapular eruptions (153 cases, 42.3%) followed by SJS (88 cases, 24.3%), DRESS (34 cases, 9.4%), TEN (21 cases, 5.8%), fixed drug eruptions (17 cases, 4.7%) and acute generalized exanthematous pustulosis (15 cases, 4.1%) [Figure - 1]. Mean onset time of rash after drug exposure for the top 5 reaction patterns were i) 15 days (range: 1-69) for maculopapular eruption, ii) 17 days (range: 5-60) for SJS, iii) 28 days (range: 7-120) for DRESS, iv) 16 days (range: 4-90) for TEN and v) 6 days (range: 2-14) for AGEP.
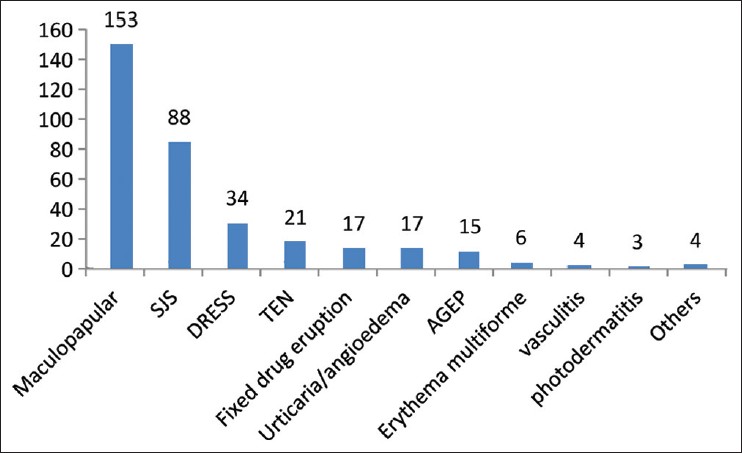 |
| Figure 1: Distribution of cutaneous adverse drug reaction patterns |
Antibiotics were the most commonly implicated drug group (146 cases, 40.3%) followed by anticonvulsants (81 cases, 22.4%), anti-gout drugs (50 cases, 13.8%) and NSAIDS (36 cases, 9.9%) [Table - 3]. The most common culprit drug was allopurinol (50 cases, 13.8%), followed by cotrimoxazole (38 cases, 10.5%), phenytoin (35 cases, 9.7%) and carbamazepine (31, 8.6%).

Drugs implicated in the various reaction patterns are shown in [Table - 4] and [Table - 5]. Maculopapular eruptions were mainly due to antibiotics (72 of 153 cases, 47.1%) and anticonvulsants (29 cases, 19.0%). Carbamazepine, allopurinol and cotrimoxazole were the three main causative drugs of SJS/TEN [Figure - 2] accounting for 21.8%, 20.9% and 12.7% respectively of the 110 cases seen whereas DRESS was mainly caused by allopurinol (15 cases, 44.1%) and phenytoin (6 cases, 17.6%). Fixed drug eruptions were mainly due to mefenamic acid (7 of 17 cases), tetracyclines (3 cases) and cotrimoxazole (3 cases). Antibiotics were responsible for 9 of the 10 urticarial reactions while NSAIDs accounted for 5 of the 7 angioedema seen. As expected, penicillins (9 out of 15 cases) accounted for the majority of acute generalized exanthematous pustulosis.
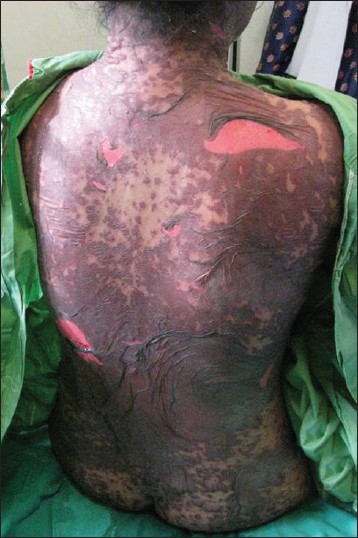 |
| Figure 2: Toxic epidermal necrolysis secondary to sulphadoxine/ pyrimethamine (Fansidar) given for malarial prophylaxis |

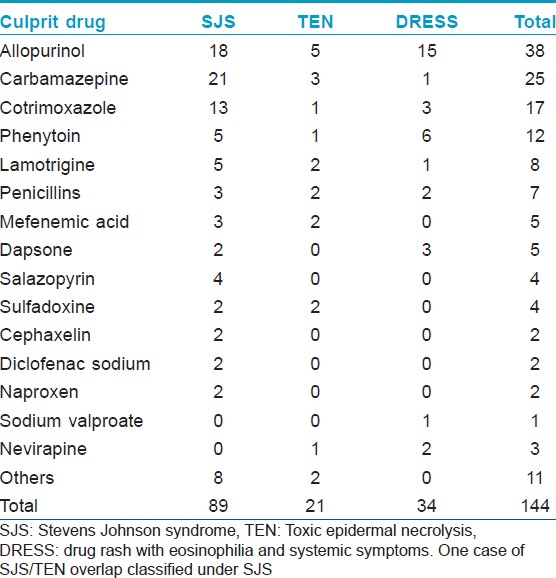
Severity
Ten patients succumbed to SCARs, 6 due to TEN [Figure - 2], 2 to SJS and 2 to DRESS. Allopurinol was responsible for 5 of the 10 deaths. Carbamazepine and cotrimoxazole were implicated in one fatality each. Mortality rates for TEN, SJS and DRESS were 28.6 %, 2.2% and 5.9% respectively.
Discussion
The frequency of cADR in a particular population is influenced by the drug utilization habit, the reaction rates of favorite drugs and pharmacogenetic traits of the population studied. Most epidemiological studies analyzed the occurrence of cADRs in inpatients and revealed a wide variation in the prevalence rate which ranged from 0.36% to 12.2%. [5],[6],[7],[8],[9],[10],[12] A meta-analysis of 39 prospective studies from US hospitals showed that 15.1% of hospitalized patients had cADR. [13] Few studies explored the rate of cADRs seen by a hospital dermatology department; cADRs accounted for 1.38% of all referrals to a university hospital department of dermatology in Denmark and 1.5% of total dermatology consultations seen at a hospital in Tunisia. [8],[14] The lower rate of cADRs (0.86%) seen in our department was probably due to referral bias where only doubtful or serious cADRs were referred to us.
We cannot fully explain the observed lower cADR rate in our Indian population. One possible reason may be due to the large number of carbamazepine- and phenytoin-induced cADRs (66 cases, 18.2%) in this study [Table - 3]. Of the 31 patients with carbamazepine-induced cADRs, 23 (74.2%) were Malay, 7 (22.6%) were Chinese and only 1 (3.2%) was Indian. Similarly, only 2 (5.7%) out of the 35 patients with phenytoin-induced cADRs were Indian compared to 29 (82.9%) Malay and 4 (11.4%) Chinese. HLA BFNx01 1502, a known genetic marker for both carbamazepine- and phenytoin- induced cADR was reported to be present in 47 of 300 (15.7%) normal Malay controls, 5.7% of 300 normal Chinese controls and in none of the 100 normal Indian controls, in a study of 21 carbamazepine-induced TEN/SJS in Malaysia. [15],[16] This disparate allelic frequency of HLA-BFNx011502 in our multi-ethnic Malaysian population may explain the high proportion of carbamazepine- and phenytoin- induced cADRs seen in our Malay patients and the lower cADR rate in our Indian population.
Descriptive analysis comparing the incidence of cADRs by gender without adjusting for the gender distribution of the population from which study samples were drawn showed conflicting results with some studies reporting a female preponderance [2],[3],[7],[8],[12] and others reporting a male preponderance. [4],[5],[6],[9] This study showed a slight male preponderance with a male to female ratio of 1.14:1. However, there was no significant difference between the two gender on the rate of cADR (male: 0.91%, female: 0.81%, P=0.28). The relative risk (RR) of male developing a cADR compared to female was 1.12 {95% confidence interval (CI): 0.91 to 1.38}.
The mean age of 39.6 years is similar to Asian studies which reported younger affected patients compared to studies from Europe and US. [4],[5],[6],[7],[8],[9],[10],[12],[13] Our study showed that the older group (20 and above) had significantly higher rate of cADR compared with the younger group; RR: 3.32 (95% CI: 2.45 to 4.51), and patients under the age of 10 were significantly less likely to have a cADR compared to teenagers (10 to 19 years old); RR: 0.41 (95% CI: 0.22 to 0.74), adults (between 20 and 59 years old): RR: 0.19 (95% CI: 0.12 to 0.31) and older adults (60 years and above): RR: 0.18 (95% CI: 0.11 to 0.31).
The clinical reaction patterns of cADR and the drugs implicated are similar to an earlier analysis of our clinic cADR registry from 2001 to 2008. [17] Our study confirmed some data already known about cADR: the most common reaction pattern was maculopapular eruptions, antibiotics were the most frequently implicated drug group, maculopapular eruptions were mainly due to antibiotics and carbamazepine was the most common cause of SJS/TEN in Asia due to a high prevalence of HLA-BFNx011502 in this population whereas allopurinol was the most main culprit drug for SJS/TEN in Europe. [2],[3],[4],[5],[6],[7],[8],[9],[12],[13],[14],[16],[17] We continue to see a large number of SCAR namely SJS/TEN (110 cases) and DRESS (34 cases) with 14 new cases of SJS/TEN [Figure - 2] and 15 new cases of DRESS from 2009 to 2010. [16] Carbamazepine, allopurinol, and cotrimoxazole remained the three main causative drugs of SJS/TEN in our population whereas allopurinol and phenytoin were responsible for the majority of DRESS seen.
Allopurinol was the single most commonly implicated drug and the majority of allopurinol-induced cADRs (78% of 50 cases) were SCAR [Table - 6] with five fatalities yielding a mortality rate of 10%. Mortality rate for SJS (2.2%) and TEN (28.6%) was comparable to those reported in literature. [10],[14],[17] We did not have any DRESS-related fatality in our initial report of 19 patients but had 2 deaths out of the 34 cases seen in this study. This observed mortality rate of 5.9% is lower than the rate reported by a Taiwanese study of 30 cases of DRESS with 3 deaths. [18],[19]

Conclusions
The low rate of cADR in our population together with the high proportion of SCAR is probably due to referral bias. Otherwise, the reaction patterns and drugs causing cADR in our population are similar to those seen in other countries. Carbamazepine, allopurinol, and cotrimoxazole were the three main causative drugs of SJS/TEN in our population.
| 1. |
Arulmani R, Rajendran AD, Suresh B. Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol 2007;65:210-6.
[Google Scholar]
|
| 2. |
Naldi L, Conforti A, Venegoni M, Troncon MG, Caputi A, Ghiotto E, et al. Cutaneous reactions to drug: An analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol 1999;48:839-46.
[Google Scholar]
|
| 3. |
Bigby M, Jick S, Arndt K. Drug-induced cutaneous reactions: A report from the Boston Collaborative Drug Surveillance Programme on 15 438 consecutive inpatients, 1975-1982. JAMA 1986;256:3358-63.
[Google Scholar]
|
| 4. |
Rahska MP, Marfatia YS. Clinical study of cutaneous drug eruptions in 200 patients. Indian J Dermatol 2008;74:74-80.
[Google Scholar]
|
| 5. |
Fiszenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand-Stocco C, Crickx B, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol 2003;149:1018-22.
[Google Scholar]
|
| 6. |
Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: Clinical pattern and causative agents: A 6 year series from Chandigarh, India. J Postgrad Med 2001;47:95-9.
[Google Scholar]
|
| 7. |
Borch JE, Andersen KE, Bindslev-Jensen C. Prevalence of acute cutaneous drug reactions in a University hospital. Acta Derm Venereol 2006;86:518-22.
[Google Scholar]
|
| 8. |
Borch JE, Andersen KE, Bindslev-Jensen C. Cutaneous adverse drug reactions seen at a University hospital Department of Dermatology. Acta Derm Venereol 2006;86:523-7.
[Google Scholar]
|
| 9. |
Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care centre in South India. Indian J Dermatol Venereol Leprol 2004;70:20-4.
[Google Scholar]
|
| 10. |
Roujeau JC, Stern RS. Severe adverse reactions to drugs. N Eng J Med 1994;333:1272-85.
[Google Scholar]
|
| 11. |
The use of the WHO-UMC system for standardised case causality assessment. Available from: http://who-umc.org/Graphics/24734.pdf. [Last accessed on 2012 Jan 26].
[Google Scholar]
|
| 12. |
Huang HY, Luo XQ, Chan LS, Cao ZH, Sun XF, XU JH. Cutaneous adverse drug reactions in a hospital-based Chinese population. Clin Exp Dermatol 2011;36:135-41.
[Google Scholar]
|
| 13. |
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A metaanalysis of prospective studies. JAMA 1998;279:1200-5.
[Google Scholar]
|
| 14. |
Zaraa I, Jones M, Trojjet S, Rouhou RC, Euch DE, Mokni M, et al. Severe adverse cutaneous drug eruptions: Epidemiological and clinical features. Int J Dermatol 2011;50:877-80.
[Google Scholar]
|
| 15. |
Lim KS, Kwan P, Chong TT. Association of HLA-B*1502 allele and carbamazepine-induced severe adverse cutaneous drug reaction among Asians, a review. Neurol Asia 2008;13:15-21.
[Google Scholar]
|
| 16. |
Chang CC, Too CL, Murad S, Hussein SH. Association of HLA-B* with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in a multiethnic Malaysian population. Int J Dermatol 2011;50:221-4.
[Google Scholar]
|
| 17. |
Ding WY, Lee CK, Choon SE. Cutaneous adverse drug reactions seen in a tertiary hospital, Johor, Malaysia . Int J Dermatol 2010;49:834-41.
[Google Scholar]
|
| 18. |
Halevy S, Ghislain PD, Mockenhaupt M, Fagot JP, Bavinck JNB, Sidoroff A, et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol 2008;58:25-32.
[Google Scholar]
|
| 19. |
Chiou CC, Yang LC, Hung SI, Chang YC, Kuo TT, Ho HC, et al. Clinicopathlogical features and prognosis of drug rash with eosinophilia and systemic symptoms: A study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol 2008;22:1044- 9.
[Google Scholar]
|
Fulltext Views
4,647
PDF downloads
2,702





