Translate this page into:
Anagen effluvium
Correspondence Address:
Amrinder J Kanwar
Department of Dermatology, Venereology and Leprology, Post Graduate Institute of Medical Education and Research, Chandigarh
India
| How to cite this article: Kanwar AJ, Narang T. Anagen effluvium. Indian J Dermatol Venereol Leprol 2013;79:604-612 |
Abstract
Disturbances of hair follicle cycling lie at the heart of most hair growth disorders, and have dramatic effects on visible hair growth and shedding. The two common disorders due to aberration in hair follicle cycling are telogen and anagen effluvium. Though a lot of literature addresses the problem of telogen effluvium, there are not many reviews on anagen effluvium or anagen hair loss. Anagen effluvium is considered synonymous with chemotherapy-induced alopecia and other causes are rarely considered. In this review, we try to discuss the etiopathogenesis, clinical presentation, differentials, and management issues in anagen effluvium. Anagen effluvium is the abrupt loss of hairs that are in their growing phase (anagen) due to an event that impairs the mitotic or metabolic activity of hair follicle. Chemotherapy, radiation and toxic chemicals, and sometimes inflammatory diseases like alopecia areata and pemphigus are also capable of diminishing the metabolic activity of hair follicles resulting in anagen hair loss. Although it is reversible, and hair regrowth occurs after a delay of 1-3 months; sometimes it can lead to permanent alopecia and can be psychologically devastating with negative impact on individual perceptions of appearance, body image, sexuality, and self-esteem. For some patients, the emotional trauma may be so severe that it may lead to discontinuing or refusing therapy that might otherwise be beneficial. In such cases, a psychosomatic approach as well as empathic consideration of the patients concerns and fears as well as the provision of practical medical-aesthetic and styling tips are equally important and can be integrated in management.Introduction
Effluvium is active hair loss of more than 100 hairs/day over a time period of 2-4 weeks, whereas alopecia is a visible reduction of hair density, which corresponds to a reduction of hair density by about 30%. On an average, the scalp is estimated to contain 100,000 hairs, of which 100 to 150 are lost daily as part of the normal hair cycle and it occurs after washing and brushing the hair. Normally, these hairs are telogen hairs. [1]
Throughout life, the hair follicle undergoes periods of cyclic growth. At any given time, 85-90% of follicles are in the anagen phase and during this phase, mitotically active matrix cells in the hair bulb differentiate and divide, resulting in hair growth. Approximately 10% of follicles are in the telogen (dormancy) phase, during which all mitotic activity is arrested and about 1% are in the catagen phase or the involution phase. The final step of the hair cycle, exogen, is when the hair is released from the follicle. [2] Multiple signaling molecules like Wnt, Sonic Hedgehog, notch, and bone morphogenic proteins have been implicated in the initial development and subsequent cycling of the hair follicle. [3],[4] The hair cycle is not synchronous throughout the scalp and the length of each phase as well as the length of the entire cycle varies with the site and the age of the patient.
Hair loss can also be classified according to the stage of the hairs shed. Anagen effluvium is the abrupt loss of hair that are in their growing phase (anagen) due to an event that impairs the mitotic or metabolic activity of hair follicle. [1] It is commonly observed as a result of radiotherapy to the head and neck or chemotherapeutic agents such as antimetabolites, alkylating agents, and mitotic inhibitors. [5],[6] These agents can impair or totally disrupt the anagen cycle and cause varying degrees of hair follicle dystrophy. Since 80-90% of scalp hairs are in the anagen phase, a large number of hairs are affected. Patients with 10% to 20% of their hair remaining after an insult almost certainly have an anagen effluvium. [1]
Anagen Effluvium
The term anagen effluvium is considered misleading because the abnormal anagen hairs in this condition are usually broken off rather than shed. Anagen effluvium is categorized into two types: The common dystrophic anagen effluvium and the loose anagen syndrome. [3]
Etiopathogenesis
Although anagen effluvium is commonly associated with chemotherapy and radiation to the head and neck [5],[6],[7],[8] other causes of anagen effluvium are: severe protein energy malnutrition, pemphigus vulgaris, [9] alopecia areata (AA), [10] and exposure to toxic agents like mercury, [11] boron, [12] thallium, [13] etc. Other medications that can rarely cause anagen effluvium include bismuth, levodopa, colchicine, and cyclosporine. [1],[5] Many systemic diseases characterized by peribulbar inflammation, such as systemic lupus erythematosus, secondary syphilis, and others, can also result in anagen arrest. [5]
The anagen phase of the hair is characterized by proliferation of the epithelial compartment, with the bulb matrix cells exhibiting the greatest proliferative activity in building up the hair shaft. Any event or insult that causes abrupt cessation of mitotic activity leads to weakening of the partially keratinized, proximal portion of the hair shaft, resulting in narrowing and subsequent breakage within the hair canal and even complete failure of hair formation. [1],[3],[5] The hair bulb itself may be damaged and the hairs may separate at the bulb and fall out. The insult is severe enough to cause a change in the rate of hair growth but does not convert the follicle to a different growth phase, as occurs in telogen effluvium. Hair shedding usually begins 1 to 3 weeks after this incident. Due to its long anagen phase, the scalp is the most common location for hair loss, while other terminal hairs are variably affected depending on the percentage of hairs in anagen. Normally, up to 90% of scalp hairs are in the anagen phase, and as such, hair loss is usually copious and results in alopecia that is quite obvious. In addition, chemotherapy given at high doses for a sufficiently long duration and with multiple exposures may also affect hairs of the beard, eyebrows, and eyelashes, as well as axillary and pubic regions. [7],[8]
The severity of hair loss after chemotherapy or radiation depends on timing, dose, and duration of the treatment and synchronization of hair cycle. When hair is in late anagen phase, during which the mitotic rate slows down spontaneously, it simply accelerates its normal path to telogen like in androgenetic alopecia. Furthermore, synchronization of hair cycles also plays a role like in androgenetic alopecia, duration of anagen is shortened and even a minor antimitotic insult can produce marked hair loss. [5],[6],[7],[8]
In anagen effluvium, only the proliferating cells in the bulb are affected, the quiescent stem cells of the bulge that are responsible for reinitiating follicle growth are spared, so hair loss is usually completely reversible. The hair follicle resumes normal cycling within a few weeks of cessation of insult/event and regrowth is apparent within 1-3 months. [1],[5],[14] The new hair may have different characteristics like graying, curling, or straightening effect, which is likely due to differential effects of chemotherapy or radiotherapy on hair follicle melanocytes and inner root sheath epithelium. [6],[7],[14]
Radiation-induced alopecia may be reversible or permanent. Radiation-induced temporary hair loss may be observed following neuroradiologically guided embolization procedures. [1] Regrowth after radiation therapy depends on type, depth, and dose-fractionation. Permanent follicular destruction is commonly seen, most likely due to irreversible damage to hair follicle stem cells. Permanent alopecia occurs with >30 Gy of deep X-rays or >50 Gy of soft X-rays. [1] Persistent radiation-induced inflammatory changes that progressively damage the stem cells may lead to scarring alopecia even after cessation of radiation therapy. [8]
Many heavy metals are capable of disrupting the formation of hair shaft by binding with the sulphydryl group of the keratins in the hair. Thallium, mercury, bismuth, copper, and cadmium are the most common metals responsible for this kind of hair loss. Mercury intoxication can occur through chronic industrial exposure, consumption of polluted water/seafood and exposure to mercury-containing antiseptics or fungicides. It can lead to hair loss with or without other symptoms of mercury poisoning. [11] Elevated mercury levels in hair, blood, or urine are considered diagnostic.
Boric acid intoxication may be due to pesticides or ingestion of household products where boric acid is a preservative. Patients may develop hair loss along with other signs and symptoms like gastrointestinal, central nervous system, and renal symptoms; a hemorrhagic diathesis; and exfoliation or bullae. [8],[12] Blood boric acid levels are elevated in affected patients.
Thallium poisoning can cause anagen hair loss within 2 to 3 weeks after exposure. [13] Patient usually presents with primarily neurologic symptoms like irritability, dysesthesia, ataxia, convulsions and coma. However, arsenic exposure does not cause hair loss, instead, arsenic is concentrated in the hair, which facilitates a diagnosis long after intoxication may have occurred. [3]
AA is a common cause of dystrophic anagen effluvium in a healthy child or adult. [10] AA is a good illustration of importance of hair cycle disturbances in hair loss. The inflammatory cell infiltrate almost exclusively attacks anagen hair bulbs; this immune attack then catapults anagen follicles into a dystrophic catagen, causing a major hair cycle disturbance and rapid shedding of the insufficiently anchored, improperly formed hair shafts. Dystrophic anagen hairs have also been observed in patients with AA incognita (AAI) by Quercetani et al. [10] and they proposed that in some patients with diffuse hair loss diagnosed as TE some patients may have AAI. Similar may be the case in pemphigus. [9] The hair follicle is a preferential target for pemphigus autoantibodies because the desmosomal proteins are overexpressed in the follicular epithelium. The hairs may come out from the lesional and perilesional areas with their outer root sheath and showing a normal anagen pattern. The normal anagen effluvium suggests a subclinical involvement of the hair follicle and can be considered a Nikolsky sign of the scalp. It may even herald pemphigus. [9]
Chemotherapy Induced Anagen Effluvium
Although many drugs have been occasionally described to produce hair loss, the relationship between drug intake and hair loss has only been proven for a few agents. The type of hair loss (i.e. telogen effluvium, anagen effluvium, or both) depends on the medication, its dosage, and patient susceptibility. [6],[7]
AE is more common and severe with combination chemotherapy than with the use of a single drug, and the severity is generally dose dependent. [7] While hair loss from anticancer therapy has traditionally been categorized as dystrophic anagen effluvium, more recently, it has been suggested that the hair follicle may respond to the same insult that is capable of stopping mitosis with both shedding patterns, i.e. dystrophic anagen effluvium and telogen effluvium. [15] Accordingly, the hair may fall out very quickly in clumps or gradually depending on the mitotic activity of the hair follicle at the moment of the insult.
Diffuse, reversible alopecia was reported in 50% of patients receiving treatment with tyrosine kinase inhibitors such as sorafenib and sunitinib [16],[17] and in 65% of patients treated with vismodegib, [18] (approved for advanced basal cell cancer) that inhibits sonic hedgehog signaling. Similarly, small molecule inhibitors of the epidermal growth factor receptor (EGFR) as well as monoclonal antibodies targeting the EGFR like cetuximab can induce a constellation of cutaneous symptoms known by the acronym PRIDE (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching and Dryness due to EGFR inhibitors). [19],[20] Mice with a targeted deletion in the EGFR gradually develop scarring alopecia. [21] These findings suggest that tyrosine kinase inhibitors and EGFR may be important molecular targets in drug-induced anagen hair loss or permanent alopecia.
The overall incidence of chemotherapy-induced hair loss is estimated to be 65%. [7] The prevalence and severity of hair loss is variable and dependent on chemotherapeutic agent and treatment protocol. There are multiple classes of anticancer drugs that can induce alopecia [Table - 1], with different frequencies of hair loss across the four major drug classes: More than 80% for antimicrotubule agents (e.g. paclitaxel), 60-100% for topoisomerase inhibitors (e.g. doxorubicin), >60% for alkylators (e.g. cyclophosphamide), and 10-50% for antimetabolites (e.g. 5-fluorouracil plus leucovorin). [7]
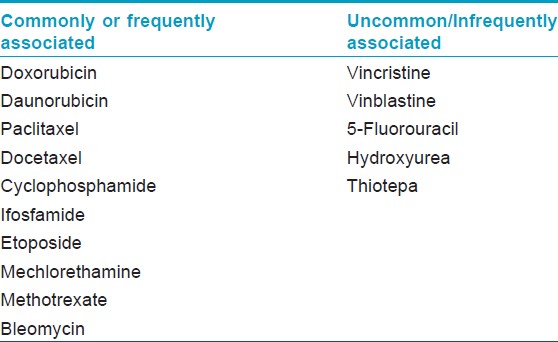
The majority of chemotherapy-induced alopecia is reversible once therapy is discontinued, with the possible exception of the epidermal growth factor receptor (EGFR) inhibitors, chemotherapy with busulfan and cyclophosphamide, and following bone marrow transplantation, [14],[22],[23] and it has also been associated with certain risk factors, including chronic graft-versus-host reaction, previous exposure to X-ray and age of patients. [6],[7],[22]
Chemotherapy-induced hair loss has a great impact on the quality of life in patients; it is considered to be one of the most traumatic factors in care of these patients. It has a negative impact on individual perceptions of appearance, body image, sexuality, and self-esteem. In a study, 47% of female patients considered hair loss to be the most traumatic aspect of chemotherapy and 8% declined chemotherapy due to fears of hair loss. [24],[25]
Loose Anagen Hair Syndrome
The loose anagen hair syndrome (LAHS) is a rare sporadic or autosomal dominant disorder with incomplete penetrance characterized by loosely anchored anagen hairs that can be easily and painlessly pulled from the scalp. It primarily affects light-haired children, with a female predominance (female to male ratio is 6:1), but rarely adults may also be affected. [26],[27],[28] LAS may result from premature and abnormal keratinization of the inner root sheath, leading to clefts between the inner and outer sheath and the hair shaft. In some patients this may result from hereditary keratin defects in the inner root sheath and/or the apposed companion layer (e.g. in families with diffuse partial woolly hair with LAHS). [29]
The typical profile of patient with LAS is a young girl with short blond hair that does not grow long, but LAS can affect children with dark hair as well. They may have sparse hair that does not grow long and have patches of dull, unruly, or matted hair with increased hair shedding. Examination shows diffuse thinning and irregular bald patches due to traumatic painless extraction of hairs. Most cases are isolated, but it can occur in hereditary or developmental disorders including coloboma, Noonan′s syndrome, and hypohidrotic ectodermal dysplasia. [30],[31],[32],[33] LAS like changes have also been associated with AIDS (AIDS trichopathy). [34]
The key to diagnosing LAHS is a high index of suspicion during the examination of an infant or toddler with diffuse nonscarring alopecia. In a study of 374 children and teenagers with hair loss, approximately 10% had LAHS, which made it a common diagnosis. [35] The differentiating features from other causes of nonscarring alopecia in children are given in [Table - 2]. [35],[36]

Unlike other nonscarring childhood hair disorders that may have associated autoimmune or hormonal findings and may require further laboratory testing based on clinical presentation and family history, most patients with LAHS have no associated laboratory test result abnormalities. [36],[37]
Microscopic examination shows anagen hair without sheath. The bulb is often misshapen, and its proximal portion often shows a visible ruffled cuticle. The hair pull test in normal children shows one or two loose anagen hairs. However, mere presence of anagen hair is not diagnostic; LAHS should only be diagnosed microscopically when there is a predominance (>50%) of loose anagen hairs on a trichogram. [26],[27],[35]
Most cases of LAHS resolve spontaneously by adulthood or adolescence; so observation is the treatment of choice; however, minoxidil therapy in infants may be a reasonable therapeutic option in patients with severe disease. [35]
Clinical Findings
Anagen effluvium can begin within days to a few weeks of the onset of chemotherapy and near complete hair loss is often well established by 2 to 3 months. However, the degree of hair loss varies from person to person and depends on a number of factors like specific agent, the dose and duration of therapy and the route of administration. Anagen effluvium is a nonscarring alopecia so the follicular ostia are intact and there is no evidence of erythema, scaling, pigmentation, and scarring. Most hair follicles are in the anagen stage at any given time; therefore, anagen alopecia affects a large percentage of the scalp. Sometimes combination of telogen effluvium and anagen effluvium can result in complete baldness.
In chemotherapy-induced hair loss, the hair loss starts from the crown and sides of the head, which could be due to the increased friction during sleep and wearing head coverings. Hair loss can be diffuse or patchy at the outset, depending on the amount of hairs in the active anagen phase. It continues for the duration of chemotherapy. Most of the patients will have regrowth, within 1 to 3 months after stopping of chemotherapy. In 60% of the cases, this regrowth can be of a different texture, thickness, color, or waviness. Associated findings can be related to the causative agent like features of heavy metal poisoning in cases due to metals like thallium, mercury etc.
Newer agents like epidermal growth factor receptor inhibitors have been linked to both scarring and nonscarring alopecia, and a number of hair changes, including changes in hair texture (brittle, fine, curly), slower growth of scalp hair, trichomegaly of eyelashes, and hypertrichosis of facial hair. Reversible hair depigmentation in the absence of alopecia has been noted to occur with the use of multitargeted receptor tyrosine kinase inhibitors including pazopanib, sunitinib, and dasatinib.
Differential Diagnosis
Anagen effluvium should be differentiated from telogen effluvium, androgenetic alopecia, trichotillomania, and other causes of nonscarring alopecia [Table - 2] and [Table - 3]. A detailed history and physical examination to identify the temporal association of possible triggers and any underlying systemic disease should be done in patients with a history of hair shedding. In some cases, further workup is required. [37],[38] The patient′s full dermatologic, systemic, and family histories should be obtained to rule out other causes of hair loss, including malnutrition, iron deficiency, endocrine and metabolic disorders, collagen vascular diseases, infections (e.g. syphilis), and widespread skin disease.

Investigations
Hair pull test and trichogram are preliminary investigations for diagnosis of anagen effluvium. Although anagen and telogen hairs can be identified with the naked eye, light microscopy is useful in doubtful cases. Anagen hairs have long indented roots covered with intact inner and outer root sheaths, and they are fully pigmented. Telogen hairs have short, club-shaped roots. They lack root sheaths and show depigmentation of the proximal part of the shaft. There is absence of the outer root in loose anagen syndrome. The characteristic finding in anagen effluvium is the tapered fracture of the hair shafts. A greater number of telogen hairs indicates a shift toward the telogen phase and suggests a probable diagnosis of telogen effluvium. Occasionally, normal anagen hairs can be easily plucked from the active phases of scarring alopecia, but they can never be plucked in non-scarring alopecia.
Although rarely required but histological changes on horizontal sections can be used to distinguish anagen effluvium from other forms of alopecia. A 4-mm punch biopsy sample of the scalp contains <15% follicles in the telogen phase. A normal anagen-to-telogen ratio in a patient with hair loss is characteristic of anagen effluvium. A finding of greater than 15% of follicles in the telogen phase indicates telogen effluvium. Moreover, follicles show no signs of inflammation, dystrophy of the inner sheath, or traction, which are helpful in the distinction of anagen effluvium from AA, androgenetic alopecia, and traction alopecia. [37],[38],[39]
Treatment
Anagen effluvium is usually reversible and appropriate hair and scalp care along with temporarily wearing of wig/hair-piece may be the most effective coping strategy required in these patients. If pharmacotherapy is to be prescribed, the goal should be to prevent or shorten the period of alopecia.
Chemotherapy-induced hair loss can at times be permanent and the studies have focused on preventing hair loss or reducing the duration of hair loss; although a number of agents have been investigated; no treatment appears to be generally effective. The major approach to minimize chemotherapy-induced hair loss has been by reducing the drug delivery to the growing hair follicle by temporarily obstructing blood flow with a tourniquet or by inducing scalp hypothermia. However, both these methods are effective in agents with a short half-life with rapid clearance of the drug and its metabolites. [40]
A scalp tourniquet consists of a pneumatic device placed around the hairline during chemotherapy infusion and inflated to a pressure greater than the systolic blood pressure. Several studies have found scalp tourniquets to be effective for preventing hair loss. [40],[41],[42] However, the data from these studies is heterogeneous with respect to different techniques used, variation in chemotherapy regimens, tourniquet pressure, sample size, and criteria to assess alopecia, rendering the data difficult to interpret or compare. Side effects observed were headache and varying degrees of nerve compression. [41]
Scalp hypothermia (scalp temperature of less than 24°C) is induced by cooling agents applied via a cooling cap or by continuous cooling of the scalp with cold air or liquid. Side effects include patient discomfort from the heavy caps, cumbersome units, and headaches and messiness. It was found useful in chemotherapy with doxorubicin, daunorubicin, paclitaxel, epirubicin, vincristine, vinblastine, actinomycin D, mechlorethamine, and combinations like cyclophosphamide, methotrexate, and fluorouracil (CMF), [43],[44],[45],[46],[47],[48],[49],[50],[51] but this approach failed with the combination of doxorubicin plus cyclophosphamide. [52] However, most published data on this technique is difficult to interpret due to use of multiple cooling systems (ice turban, gel packs, cool caps, thermocirculator, room air conditioner), variable chemotherapy regimens (single versus combined agents), small study populations, and varying definitions of alopecia. [6],[7],[41],[42],[43],[44],[45],[46],[47],[48],[49],[50],[51],[52],[53] Scalp may act as a sanctuary for circulating malignant cells so patients with leukemia, lymphoma, and other hematologic malignancies are generally not suitable candidates for these procedures. Use of these techniques is controversial in patients with non-hematological malignancies who are undergoing curative chemotherapy. [51]
Pharmacologic interventions-Preliminary studies have suggested that a variety of both small molecules and biologic agents may reduce or prevent alopecia by protecting the hair bulb from the damaging effects of chemotherapy [Table - 4]. Unfortunately, none of these agents have demonstrated adequate activity in large, randomized clinical trials to justify their general usage. In humans, AS101 and minoxidil were able to reduce the severity or shorten the duration of chemotherapy-induced alopecia, but could not prevent the hair loss. [55],[69],[70] Calcitriol, calcineurin inhibitors, and interleukin-1 can prevent or ameliorate chemotherapy-induced alopecia in various animal models, but clinical trials of topical calcitriol and calcipotriol have shown lack of efficacy. [71],[72] Topical dexamethasone, calcitriol, and estradiol promote the regrowth of normally pigmented hair shafts in mouse models of chemotherapy-induced alopecia. [73] Recent in vitro evidence suggests that keratinocyte growth factor may exert protective effects on chemotherapy-damaged human scalp hair follicles. [74] Hopefully, localized hair-saving treatment may be developed that does not negatively affect chemotherapy efficacy. An ideal therapeutic agent should selectively target the hair follicle without interfering with the efficacy of chemotherapy. Chemotherapeutic agents are used in combination so an effective strategy would require agents that are effective for different chemotherapeutics with distinct mechanisms of action. Moreover, variations in patient characteristics must also be taken into account, as the pattern of chemotherapy-induced hair loss is patient-specific.
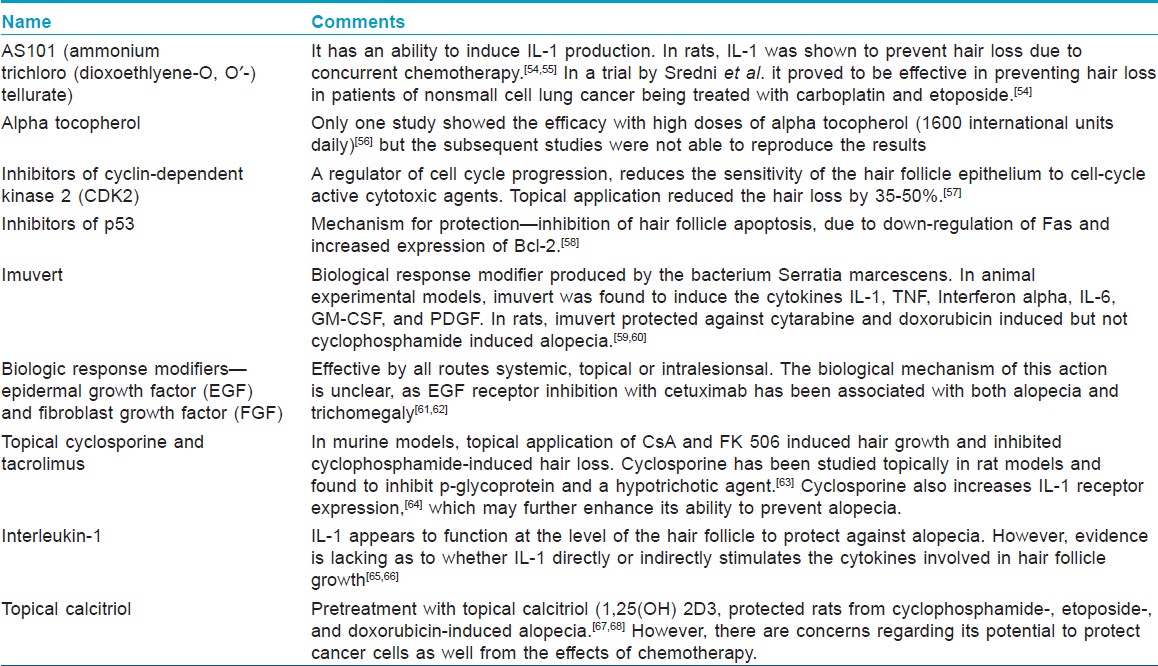
Since an effective pharmacologic management of chemotherapy-induced alopecia still eludes us. Patient education is very important in anagen effluvium as drug-induced alopecia can be psychologically devastating to a patient. Anticipating hair loss, coming to terms with the inevitability of hair loss and maintaining a proactive disposition are the key steps in successfully coping with chemotherapy-induced hair loss. Patients should also be reassured that the hair loss is temporary and normal hair growth will occur a few weeks after completion of treatment although the color or texture of the regrowing hair may differ from those of the original hair.
Recommendations for hair care include: Avoiding physical or chemical trauma to the hair (e.g. bleaching, coloring, perming, using curling irons, or hot rollers). Using a satin pillowcase, which is less likely to attract and catch fragile hair; using a soft brush, washing hair only as often as necessary; and using a gentle shampoo. Cutting hair short or shaving hair as it looks fuller than long hair, and when the hair is shed, it is less noticeable when it is short. Moreover, hair that has been cut short may help patients to ease the transition to total alopecia. Shaving the head may be easier for securing a wig or hairpiece. [75]
Patients can be encouraged to plan for an appropriate head covering in advance. The use of a head covering/hairpiece or wig is a very personal decision. Men often view it as a normal and inevitable consequence of treatment but for women it is difficult. Depending on preference, using a wig or any other type of head covering until the hair regrows is an effective way of dealing with this condition, while at the same time it can protect the scalp from sun and cold exposure. [76],[77],[78]
Conclusion
Anagen effluvium occurs when metabolic and mitotic activity of the follicular epithelium is rapidly suppressed. Chemotherapy, radiation, toxic chemicals and sometimes inflammatory diseases like AA and pemphigus can cause anagen hair loss. However it must be stressed that anagen effluvium is reversible, with hair regrowth typically occurring after a delay of 1-3 months when the follicle resumes its normal activity after withdrawal of the antimitotic factors. Although in some cases, hair regrows despite continued or maintenance therapy. The major medical approach to prevent or minimize chemotherapy-induced hair loss remains scalp cooling, while topical minoxidil may speed up hair regrowth. Since chemotherapy-induced hair loss cannot be reliably prevented, it is recommended that a management scheme be devised in advance which focuses on treatment expectations and making patients as comfortable as possible with their appearance before, during, and after anticancer therapy.
| 1. |
Trüeb RM. Diffuse hair loss. In: Blume-Peytavi U, Tosti A, Whiting DA, Trüeb RM, editors. Hair Growth and Disorders. Berlin: Springer; 2008. p. 259-72.
[Google Scholar]
|
| 2. |
Vogt A, McElvee K, Blume-Peytavi U. Biology of the hair follicle. In: Blume-Peytavi U, Tosti A, Whiting D, Trüeb R, editors. Textbook on hair - From basic science to clinical application. Berlin: Springer Verlag; 2008. p. 1-22.
[Google Scholar]
|
| 3. |
Paus R, Olsen EA, Messenger AG. Hair Growth Disorders. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Zller AS, Leffell DJ, editors. Fitzpatrick′s Dermatology in General Medicine. 7 th ed., vol. 2. New York: McGraw-Hill; 2008. p. 753-77.
[Google Scholar]
|
| 4. |
Sato N, Leopold PL, Crystal RG. Effect of adenovirus-mediated expression of Sonic hedgehog gene on hair regrowth in mice with chemotherapy-induced alopecia. J Natl Cancer Inst 2001;93:1858-64.
[Google Scholar]
|
| 5. |
Sperling LC. Hair and systemic disease. Dermatol Clin 2001;19:711-26.
[Google Scholar]
|
| 6. |
Tosti A, Pazzaglia M. Drug reactions affecting hair: Diagnosis. Dermatol Clin 2007;25:223-31.
[Google Scholar]
|
| 7. |
Trüeb R. Chemotherapy-induced Hair Loss. Skin Therapy Lett 2010;15:5-7.
[Google Scholar]
|
| 8. |
Sinclair R, Grossman KL, Kvedar JC. Anagen hair loss, in Disorders of Hair Growth: Diagnosis and Treatment. In: Olsen EA, editor. McGraw-Hill: New York; 2002.p. 275.
[Google Scholar]
|
| 9. |
Delmonte S, Semino MT, Parodi A, Rebora A. Normal anagen effluvium: A sign of pemphigus vulgaris. Br J Dermatol 2000;142:1244-5.
[Google Scholar]
|
| 10. |
Quercetani R, Rebora AE, Fedi MC, Carelli G, Mei S, Chelli A, et al. Patients with profuse hair shedding may reveal anagen hair dystrophy: A diagnostic clue of alopecia areata incognita. J Eur Acad Dermatol Venereol 2011;25:808-10.
[Google Scholar]
|
| 11. |
Elhassani SB. The many faces of methyl mercury poisoning. J Toxicol Clin Toxicol 1982;19:875-906.
[Google Scholar]
|
| 12. |
Stein KM, Odom RB, Justice GR, Martin GC. Toxic alopecia from ingestion of boric acid. Arch Dermatol 1973;108:95-7.
[Google Scholar]
|
| 13. |
Bank WJ, Pleasure DE, Suzuki K, Nigro M, Katz R. Thallium poisoning. Arch Neurol 1972;26:456-64.
[Google Scholar]
|
| 14. |
Vowels M, Chan LL, Giri N, Russell S, Lam-Po-Tang R. Factors affecting hair regrowth after bone marrow transplantation. Bone Marrow Transplant 1993;12:347-50.
[Google Scholar]
|
| 15. |
Trueb RM. Chemotherapy-induced anagen effluvium: Diffuse or patterned? Dermatology 2007;215:1-2.
[Google Scholar]
|
| 16. |
Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol 2008;144:886-92.
[Google Scholar]
|
| 17. |
Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol 2009;60:299-305.
[Google Scholar]
|
| 18. |
O′Bryan KW, Ratner D. The role of targeted molecular inhibitors in the management of advanced nonmelanoma skin cancer. Semin Cutan Med Surg 2011;30:57-61.
[Google Scholar]
|
| 19. |
Donovan JC, Ghazarian DM, Shaw JC. Scarring alopecia associated with use of the epidermal growth factor receptor inhibitor gefitinib. Arch Dermatol 2008;144:1524-5.
[Google Scholar]
|
| 20. |
Graves JE, Jones BF, Lind AC, Heffernan MP. Nonscarring inflammatory alopecia associated with the epidermal growth factor receptor inhibitor gefitinib. J Am Acad Dermatol 2006;55:349-53.
[Google Scholar]
|
| 21. |
Murillas R, Larcher F, Conti CJ, Santos M, Ullrich A, Jorcano JL. Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. EMBO J 1995;14:5216-23.
[Google Scholar]
|
| 22. |
Palamaras I, Misciali C, Vincenzi C, Robles WS, Tosti A. Permanent chemotherapy-induced alopecia: A review. J Am Acad Dermatol 2011;64:604-6.
[Google Scholar]
|
| 23. |
Baker BW, Wilson CL, Davis AL, Spearing RL, Hart DN, Heaton DC, et al. Busulphan/cyclophosphamide conditioning for bone marrow transplantation may lead to failure of hair regrowth. Bone Marrow Transplant 1991;7:43-7.
[Google Scholar]
|
| 24. |
McGarvey EL, Baum LD, Pinkerton RC, Rogers LM. Psychological sequelae and alopecia among women with cancer. Cancer Pract 2001;9:283-9.
[Google Scholar]
|
| 25. |
Munstedt K, Manthey N, Sachsse S, Vahrson H. Changes in self-concept and body image during alopecia induced cancer chemotherapy. Support Care Cancer 1997;5:139-43.
[Google Scholar]
|
| 26. |
Price VH, Gummer CL. Loose anagen syndrome. J Am Acad Dermatol 1989;20:249-56.
[Google Scholar]
|
| 27. |
Hamm H, Traupe H. Loose anagen hair of childhood: The phenomenon of easily pluckable hair. J Am Acad Dermatol 1989;20 (2 Pt 1):242-8.
[Google Scholar]
|
| 28. |
Tosti A, Piraccini BM. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol 2002;138:521-2.
[Google Scholar]
|
| 29. |
Chapalain V, Winter H, Langbein L, Le Roy JM, Labrèze C, Nikolic M, et al. Is the loose anagen hair syndrome a keratin disorder? A clinical and molecular study. Arch Dermatol 2002;138:501-6.
[Google Scholar]
|
| 30. |
Hansen LK, Brandrup F, Clemmensen O. Loose anagen syndrome associated with colobomas and dysmorphic features. Clin Dysmorphol 2004;13:31-2.
[Google Scholar]
|
| 31. |
Murphy MF, McGinnity FG, Allen GE. New familial association between ocular coloboma and loose anagen syndrome. Clin Genet 1995;47:214-6.
[Google Scholar]
|
| 32. |
Tosti A, Misciali C, Borrello P, Fanti PA, Bardazzi F, Patrizi A. Loose anagen hair in a child with Noonan′s syndrome. Dermatologica 1991;182:247-9.
[Google Scholar]
|
| 33. |
Azon-Masoliver A, Ferrando J. Loose anagen hair in hypohidrotic ectodermal dysplasia. Pediatr Dermatol 1996;13:29-32.
[Google Scholar]
|
| 34. |
Sadick NS. Clinical and laboratory evaluation in AIDS trichopathy. Int J Dermatol 1993;32:33-8.
[Google Scholar]
|
| 35. |
Cantatore-Francis JL, Orlow SJ. Practical guidelines for evaluation of loose anagen hair syndrome. Arch Dermatol 2009;145:1123-8.
[Google Scholar]
|
| 36. |
Mandt A, Vogt A, Blume-Peytavi U. Differential diagnosis of hair loss in children. J Dtsch Dermatol Ges 2004;2:399-411.
[Google Scholar]
|
| 37. |
Hillmann K, Blume-Peytavi U. Diagnosis of hair disorders. Semin Cutan Med Surg 2009;28:33-8.
[Google Scholar]
|
| 38. |
Tosti A, Gray J. Assessment of hair and scalp disorders. J Investig Dermatol Symp Proc 2007;12:23-7.
[Google Scholar]
|
| 39. |
Blume-Peytavi U, Vogt A. Current standards in the diagnostics and therapy of hair diseases - hair consultation. J Dtsch Dermatol Ges 2011;9:394-410.
[Google Scholar]
|
| 40. |
Wang J, Lu Z, Au JL. Protection against chemotherapy-induced alopecia. Pharm Res 2006;23:2505-14.
[Google Scholar]
|
| 41. |
Hussein AM. Chemotherapy-induced alopecia: New developments. South Med J 1993;86:489-96.
[Google Scholar]
|
| 42. |
Cline BW. Prevention of chemotherapy-induced alopecia: A review of the literature. Cancer Nurs 1984;7:221-8.
[Google Scholar]
|
| 43. |
Edelstyn GA, MacDonald M, MacRae KD. Doxorubicin-induced hair loss and possible modification by scalp cooling. Lancet 1997;2:253-4.
[Google Scholar]
|
| 44. |
Giaccone G, Di Giulio F, Morandini MP, Calciati A. Scalp hypothermia in the prevention of doxorubicin-induced hair loss. Cancer Nurs 1988;11:170-3.
[Google Scholar]
|
| 45. |
Kennedy M, Packard R, Grant M, Padilla G, Presant C, Chillar R. The effects of using Chemocap on occurrence of chemotherapy-induced alopecia. Oncol Nurs Forum 1983;10:19-24.
[Google Scholar]
|
| 46. |
Macduff C, Mackenzie T, Hutcheon A, Melville L, Archibald H. The effectiveness of scalp cooling in preventing alopecia for patients receiving epirubicin and docetaxel. Eur J Cancer Care (Engl) 2003;12:154-61.
[Google Scholar]
|
| 47. |
Parker R. The effectiveness of scalp hypothermia in preventing cyclophosphamide-induced alopecia. Oncol Nurs Forum 1987;14:49-53.
[Google Scholar]
|
| 48. |
Ron IG, Kalmus Y, Kalmus Z, Inbar M, Chaitchik S. Scalp cooling in the prevention of alopecia in patients receiving depilating chemotherapy. Support Care Cancer 1997;5:136-8.
[Google Scholar]
|
| 49. |
Witman G, Cadman E, Chen M. Misuse of scalp hypothermia. Cancer Treat Rep 1981;65:507-8.
[Google Scholar]
|
| 50. |
Forsberg SA. Scalp cooling therapy and cytotoxic treatment. Lancet 2001;357:1134.
[Google Scholar]
|
| 51. |
Grevelman EG, Breed WP. Prevention of chemotherapy-induced hair loss by scalp cooling. Ann Oncol 2005;16:352-8.
[Google Scholar]
|
| 52. |
Tran D, Sinclair RD, Schwarer AP, Chow CW. Permanent alopecia following chemotherapy and bone marrow transplantation. Australas J Dermatol 2000;41:106-8.
[Google Scholar]
|
| 53. |
Middleton J, Franks D, Buchanan RB, Hall V, Smallwood J, Williams CJ. Failure of scalp hypothermia to prevent hair loss when cyclophosphamide is added to doxorubicin and vincristine. Cancer Treat Rep 1985;69:373-5.
[Google Scholar]
|
| 54. |
Sredni B, Albeck M, Tichler T, Shani A, Shapira J, Bruderman I, et al. Bone marrow-sparing and prevention of alopecia by AS101 in non-small-cell lung cancer patients treated with carboplatin and etoposide. J Clin Oncol 1995;13:2342-53.
[Google Scholar]
|
| 55. |
Sredni B, Xu RH, Albeck M, Gafter U, Gal R, Shani A, et al. The protective role of the immunomodulator AS101 against chemotherapy-induced alopecia studies on human and animal models. Int J Cancer 1996;65:97-103.
[Google Scholar]
|
| 56. |
Wood LA. Possible prevention of adriamycin-induced alopecia by tocopherol. N Engl J Med 1985;312:1060.
[Google Scholar]
|
| 57. |
Davis ST, Benson BG, Bramson HN, Chapman DE, Dickerson SH, Dold KM, et al. Prevention of chemotherapy-induced alopecia in rats by CDK inhibitors. Science 2001;291:134-7.
[Google Scholar]
|
| 58. |
Botchkarev VA, Komarova EA, Siebenhaar F, Botchkareva NV, Komarov PG, Maurer M, et al. p53 is essential for chemotherapy-induced hair loss. Cancer Res 2000;60:5002-6.
[Google Scholar]
|
| 59. |
Jiménez JJ, Huang HS, Yunis AA. Treatment with ImuVert/N-acetylcysteine protects rats from cyclophosphamide/cytarabine-induced alopecia. Cancer Invest 1992;10:271-6.
[Google Scholar]
|
| 60. |
Hussein AM, Jimenez JJ, McCall CA, Yunis AA. Protection from chemotherapy-induced alopecia in a rat model. Science 1990;249:1564-6.
[Google Scholar]
|
| 61. |
Jimenez JJ, Yunis AA. Protection from 1-beta-D-arabinofuranosylcytosine-induced alopecia by epidermal growth factor and fibroblast growth factor in the rat model. Cancer Res 1992;52:413-5.
[Google Scholar]
|
| 62. |
Dueland S, Sauer T, Lund-Johansen F, Ostenstad B, Tveit KM. Epidermal growth factor receptor inhibition induces trichomegaly. Acta Oncol 2003;42:345-6.
[Google Scholar]
|
| 63. |
Hussein AM, Stuart A, Peters WP. Protection against chemotherapy-induced alopecia by cyclosporin A in the newborn rat animal model. Dermatology 1995;190:192-6.
[Google Scholar]
|
| 64. |
Degiannis D, Stein S, Czarnecki M, Raskova J, Raska K Jr. Cyclosporine-induced enhancement of interleukin 1 receptor expression by PHA-stimulated lymphocytes. Transplantation 1990;50:1074-6.
[Google Scholar]
|
| 65. |
Jimenez JJ, Wong GH, Yunis AA. Interleukin 1 protects from cytosine arabinoside-induced alopecia in the rat model. FASEB J 1991;5:2456-8.
[Google Scholar]
|
| 66. |
Jimenez JJ, Sawaya ME, Yunis AA. Interleukin 1 protects hair follicles from cytarabine (ARA-C)-induced toxicity in vivo and in vitro. FASEB J 1992;6:911-3.
[Google Scholar]
|
| 67. |
Jimenez JJ, Yunis AA. Protection from chemotherapy-induced alopecia by 1,25-dihydroxyvitamin D3. Cancer Res 1992;52:5123-5.
[Google Scholar]
|
| 68. |
Jimenez JJ, Alvarez E, Bustamante CD, Yunis AA. Pretreatment with 1,25(OH) 2D3 protects from Cytoxan-induced alopecia without protecting the leukemic cells from Cytoxan. Am J Med Sci 1995;310:43-7.
[Google Scholar]
|
| 69. |
Rodriguez R, Machiavelli M, Leone B, Romero A, Cuevas MA, Langhi M, et al. Minoxidil (Mx) as a prophylaxis of doxorubicin--induced alopecia. Ann Oncol 1994;5:769-70.
[Google Scholar]
|
| 70. |
Duvic M, Lemak NA, Valero V, Hymes SR, Farmer KL, Hortobagyi GN, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol 1996;35:74-8.
[Google Scholar]
|
| 71. |
Bleiker TO, Nicolaou N, Traulsen J, Hutchinson PE. ′Atrophic telogen effluvium′ from cytotoxic drugs and a randomized controlled trial to investigate the possible protective effect of pretreatment with a topical vitamin D analogue in humans. Br J Dermatol 2005;153:103-12.
[Google Scholar]
|
| 72. |
Hidalgo M, Rinaldi D, Medina G, Griffin T, Turner J, Von Hoff DD. A phase I trial of topical topitriol (calcitriol, 1,25-dihydroxyvitamin D3) to prevent chemotherapy-induced alopecia. Anticancer Drugs 1999;10:393-5.
[Google Scholar]
|
| 73. |
Hendrix S, Handjiski B, Peters EM, Paus R. A guide to assessing damage response pathways of the hair follicle: Lessons from cyclophosphamide-induced alopecia in mice. J Invest Dermatol 2005;125:42-51.
[Google Scholar]
|
| 74. |
Braun S, Krampert M, Bodó E, Kümin A, Born-Berclaz C, Paus R, et al. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci 2006;119(Pt 23):4841-9.
[Google Scholar]
|
| 75. |
Mayo Clinic. Chemotherapy and hair loss: What to expect during treatment. Available from: http://www.mayoclinic.com/health/hair-loss/CA00037. [Last accessed on 2012 Jun10].
[Google Scholar]
|
| 76. |
Merial-Kieny C, Nocera T, Mery S. Medical corrective make-up in post- chemotherapy. Ann Dermatol Venereol 2008;1:25-8.
[Google Scholar]
|
| 77. |
Rosman S. Cancer and stigma: Experience of patients with chemotherapy-induced alopecia. Patient Educ Couns 2004;52:333-9.
[Google Scholar]
|
| 78. |
Jimenez JJ, Roberts SM, Mejia J, Mauro LM, Munson JW, Elgart GW, et al. Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones 2008;13:31-8.
[Google Scholar]
|
Fulltext Views
22,036
PDF downloads
4,481





