Translate this page into:
Analysis of Neisseria gonorrhoeae susceptibility trends (MIC creep) from North India: A 15-years’ experience!
Corresponding author: Dr. Seema Sood, Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India. seemalsood@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sood S, Gupta S, Verma R, Singh R, Agrawal SK, Mahajan N, et al. Analysis of Neisseria gonorrhoeae susceptibility trends (MIC creep) from North India: A 15-years’ experience! Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1096_2024
Abstract
Background
Neisseria gonorrhoeae (NG) is a highly transformable strict human pathogen with the potential to evolve into a ‘superbug’ Resource-limited settings like ours rely on the syndromic diagnostic approach recommended by the WHO and adopted by the National AIDS Control Organisation (NACO) for the management of sexually transmitted infections (STIs). The ‘Grey kit” comprising of cefixime 400 mg and azithromycin 1 gm is recommended for genitourinary discharge in India.
Aim
The objective of this study was to examine putative changes in minimum inhibitory concentration (MICs) of ceftriaxone/cefixime and azithromycin during a ∼15-year period to assess the need for dosage adjustment.
Methods
All clinical isolates of NG obtained from patients attending the STI clinic of our hospital were included in the study. The MIC for penicillin, tetracycline, ciprofloxacin, ceftriaxone, cefixime, azithromycin, and spectinomycin was determined using the E-test method. The results were interpreted according to the Calibrated Dichotomous Sensitivity (CDS) criteria except for azithromycin (up to 2011) and cefixime, where Clinical & Laboratory Standards Institute (CLSI) guidelines were used. We analysed our data by years (2008-2012 vs. 2013-2017) and examined the limited data available thereafter until June 2023 (in light of COVID). MIC50 and MIC90 (MIC values that stop the growth of 50% and 90% of bacterial isolates respectively) data were analysed for determination of MIC creep.
Results
A total of 183 NG isolates were collected during the study period (151 during 2008-2017 and 32, 2018 onwards). All isolates were from male patients presenting with urethritis. High resistance levels were observed for penicillin, tetracycline, and ciprofloxacin, but decreased susceptibility to ceftriaxone or cefixime remained (<10%), as did resistance to azithromycin (<5%). Notably, no ceftriaxone resistance was detected over the ∼15-year period, and no significant increase in MIC (MIC creep) was observed for ceftriaxone and azithromycin. Only two isolates showed concurrent resistance to azithromycin and decreased susceptibility to ceftriaxone. All isolates were susceptible to spectinomycin.
Limitations
The clinical isolates were obtained from a single site, and the numbers were limited. There is a paucity of data during the COVID-19 pandemic due to limited clinical services being offered.
Conclusion
There appears to be no immediate threat to the therapies currently being used in syndromic management for genitourinary discharge. However, MIC-based monitoring of crucial antimicrobials is imperative.
Keywords
Gonorrhoea
antimicrobial resistance
MIC creep
ceftriaxone
azithromycin
Introduction
Gonorrhoea is one of the most prevalent STIs worldwide and a global health problem. Its significance has further increased due to its syndemic relationship with HIV. However, determining the incidence and prevalence rates of gonorrhoea proves challenging due to a scarcity of resources in areas with high prevalence.1 Some available estimates of incidence suggest that approximately 82 million new cases of gonorrhoea occur among adults aged 15-49 years globally each year.2 The continued spread of gonococcal infections, is further exacerbated by its ability to develop resistance to antibiotics. Over the past two decades, strains of N. gonorrhoeae (NG) with high levels of resistance against several antimicrobial agents, including penicillin, tetracycline, and quinolones, have been reported from various countries.3-6 The emergence of resistance in NG has been attributed to high antimicrobial exposure due to increased general consumption, indiscriminate/incorrect use of antimicrobials, and lack of follow-up and surveillance of antimicrobial resistance (AMR), leading to bystander selection (direct/indirect). Further complicating the matter is the circulation of suboptimal therapeutic preparations.7 Resistance to extended-spectrum cephalosporins (ESC), recommended as the first-line antibiotics for the treatment of uncomplicated gonococcal infections, is a significant global concern and may complicate the management of gonorrhoea.7-9 Further, strains with decreased susceptibility (DS) to ceftriaxone also have high minimum inhibitory concentrations (MICs) for penicillin, tetracycline, and quinolones, which rules out their reintroduction in the current era.10,11
Key events in the evolution of NG have been the emergence of penicillin resistance and the progressive increase of ceftriaxone MIC, which has been described as ‘MIC creep’12 The objective of this study was to examine the dynamics of NG AMR patterns and their MIC trends over a continuous 10-year period spanning 2008 to 2017. Thereafter, limited data was examined from 2018 onwards until June 2023 (due to COVID). We used MIC50 and MIC 90 (MIC values that stop the growth of 50% and 90% of bacterial isolates respectively) data to predict ‘MIC creep” for the antibiotics over this time period to evaluate the need for dosage adjustment/escalation for ESCs and azithromycin, the two crucial antibiotics. This study provided a valuable opportunity to assess the prevalence and patterns of AMR in clinical NG isolates over the given period and also evaluate any emerging resistance. No study from India has investigated the ‘MIC creep’ of various antimicrobials in clinical gonococcal isolates so far, to the best of our knowledge.
Methods
Bacterial isolates
All gonococcal isolates were obtained from clinical samples of STI clinic attendees between January 2008 and June 2023 at a 3194 bedded tertiary care hospital in Delhi. Patients with urethral (for males) and cervical/vaginal (for females) discharge were included in the study. Further, patients with a history of STI, symptomatic partners, positive risk factors, or partners with positive risk factors were also included in the study. Urethral and endocervical swabs were collected from male and female patients, respectively, by trained physicians as per standard protocol. The standard methods, including microscopy following Gram staining, culture, and biochemical tests/ MALDI-TOF (matrix-assisted laser desorption/ionisation- time-of-flight) mass spectrometry were employed to identify gonococcal isolates. The isolates were preserved in 20% glycerol broth by deep freezing at -70°C (-86°C ULT Freezer, Model 991; Thermo Scientific) and lyophilised (Free Zone Triad Freeze dry system: Model – 7400030 from Labcono). The stocked isolates were first revived on chocolate agar plates by incubating at 36°C with 5 % CO2 and then tested for antimicrobial susceptibility.
Antimicrobial susceptibility testing (AST)
MICs of penicillin, tetracycline, ciprofloxacin, ceftriaxone, spectinomycin, and azithromycin for all isolates were determined using E-test strips (Biomerieux, France) and MIC-test strips (Liofilchem, Itlay) as per manufacturer’s instructions. Results were interpreted as per the breakpoint criteria of the Calibrated Dichotomous Sensitivity (CDS) technique.13 Additionally, β-lactamase producers were tested using the chromogenic nitrocefin discs (BD, USA). For quality control, the WHO NG reference strains (F, G, and K-P) were used. In addition to internal quality control, our lab has been participating in the External Quality Assessment Scheme (EQAS) program of the WHO Global Gonococcal Antimicrobial Surveillance Program (GASP) conducted by Regional Reference Laboratory (RRL), Vardhman Mahavir Medical College & Safdarjung Hospital, New Delhi since 2005. Breakpoints were determined in accordance with the CDS criteria: MICs of ≥1 µg/mL for penicillin, ≥16 µg/mL for plasmid-mediated tetracycline resistant NG, 2-8 µg/mL for chromosomally mediated tetracycline resistance, ≥1µg/mL for ciprofloxacin, ≥1 µg/mL for azithromycin and ≥128µg/mL for spectinomycin were considered resistant. For ceftriaxone, MICs of 0.06-0.25 µg/mL were classified as DS.13 However, for azithromycin, CDS breakpoints became available in 2011. Prior to this, CLSI breakpoints were used. MIC testing for cefixime was initiated in 2017, and a MIC > 0.125 µg/mL was designated as less susceptible (CLSI guidelines). The isolates were categorised into nine resistant phenotypes [Figure 1].
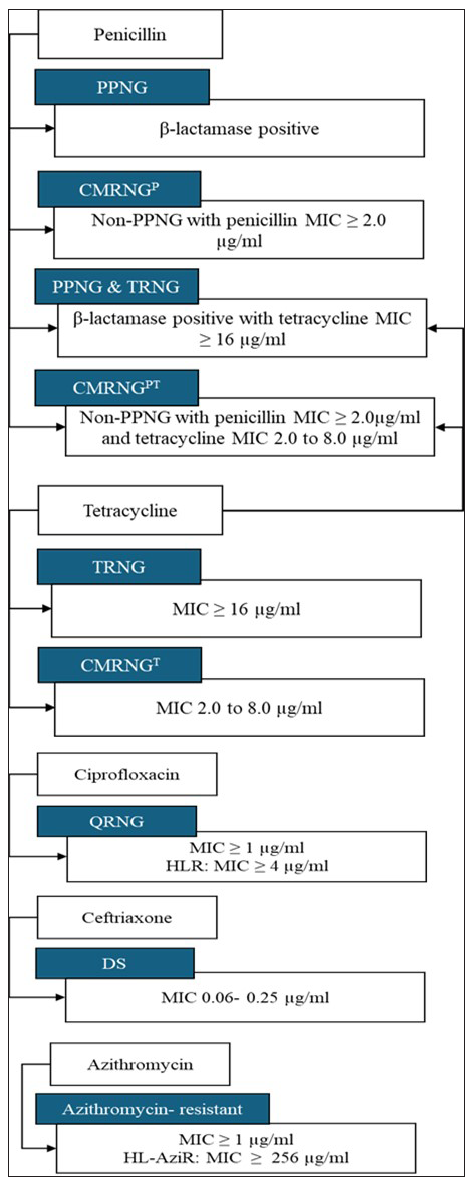
- Resistant phenotypic categories in Neisseria gonorrhoeae. The isolates were divided into nine resistant phenotypes. PPNG: Penicillinase-producing Neisseria gonorrhoeae, CMRNG(P): Chromosomally mediated penicillin resistant N. gonorrhoeae, TRNG: Plasmid-mediated tetracycline resistant N. gonorrhoeae, CMRNG(PT): Chromosomally mediated penicillin and tetracycline resistant N. gonorrhoeae, CMRNG(T): Chromosomally mediated tetracycline resistant N. gonorrhoeae, QRNG: Quinolone resistant N. gonorrhoeae, HLR: High-level resistance, DS: Decreased susceptibility to ceftriaxone
This study was approved by the institute ethics committee (Ref. No. IEC/NP-A-52/2006 and OP-18/2013 and RP-01/05.08.2013 and IESC/T-429/28.11.14)
Data analysis
The AMR trend was analysed year-wise and simultaneous examination of the MIC change for each antimicrobial was done. The statistical significance of these trends was evaluated using a non-parametric trend test. Descriptive analysis was done based on the frequency and the MIC50 and MIC90 data of each antimicrobial, categorised by the years (2008 to 2012 versus 2013 to 2017), to examine the potential changes in MICs during the emergence of AMR.12 MIC50 was the median MIC, whereas MIC90 was the 90th percentile for each antimicrobial as defined by the frequency distribution. Data were analysed using STATA/SE 17 for Windows (StataCorp, LLC). Apart from the 10-year period (2008 to 2017), data were also analysed from January 2018 to June 2023, although we did not have any isolates obtained during 2020-2021 due to the COVID-19 pandemic.
Results:
Year-wise antimicrobial resistance profile of Neisseria gonorrhoeae isolates
From January 2008 to December 2017, a total of 151 gonococcal isolates were confirmed by culture and biochemical tests or MALDI-TOF. Overall, 109 (72.2%) isolates exhibited penicillin resistance, and of these, 84 (55.6%) were plasmid-mediated, 46 (30.5%) were chromosomally mediated tetracycline-resistant, while 47 (31.1%) were plasmid-mediated tetracycline resistant. Resistance to quinolone was seen in 143 (94.7%) strains. An increase in the prevalence of gonococcal resistance to penicillin, tetracycline, and ciprofloxacin was observed during the study period. Between 2008 and 2017, the prevalence of penicillinase-producing Neisseria gonorrhoeae (PPNG) increased significantly from 47.2% to 67.7% (p-value = 0.012). Between 2008 and 2017, there was a significant increase in the prevalence of high-level resistance (HLR) to ciprofloxacin from 58.4% to 85.5% (p-value < 0.001) [Table 1]. Six (4%) isolates exhibited DS to ceftriaxone, and 4 (2.6%) were azithromycin resistant. No resistant strains to ceftriaxone were found during the 10-year period. Two isolates exhibited concurrent resistance to azithromycin and DS to ceftriaxone. All the isolates were susceptible to spectinomycin. No multi-drug resistant (MDR) and extensively drug-resistant (XDR) NG were described during this period.
| Resistant phenotype | 2008-2017 (151) | 2008-2012 (89) | 2013-2017 (62) | P value |
|---|---|---|---|---|
| CMRNGP | 25 (16.5%) | 14 (15.7%) | 11 (17.7%) | 0 .744 |
| PPNG | 84 (55.6%) | 42 (47.2%) | 42 (67.7%) | 0.012 |
| CMRNGT | 46 (30.4%) | 22 (24.7%) | 24 (38.7%) | 0.066 |
| TRNG | 47 (31.1 %) | 25 (28%) | 22 (35.5%) | 0.334 |
| QRNG | 143 (94.7%) | 84 (94.3%) | 59 (95.1%) | 0.833 |
| HLR | 105 (75.5%) | 52 (58.4%) | 53 (85.5%) | <0.001 |
CMRNG(P): Chromosomally mediated penicillin resistant N. gonorrhoeae CMRNG(T): Chromosomally mediated tetracycline resistant N. gonorrhoeae
MIC profiles of antimicrobials
The increase in MIC90 for penicillin and MIC50 for tetracycline over time (p>0.05) were marginally statistically significant. Over time, MIC90 for ciprofloxacin increased from 2 µg/mL to 32 µg/mL from 2008-2012 to 2013-2017 [Table 2]. During the 10-year study period, we did not observe any resistance to spectinomycin, nor did we see any significant change in the MIC of the antimicrobial. Further, no MIC creep was observed for ceftriaxone, as demonstrated by the year-wise trend assessment of MIC50 and MIC90 data [Figure 2 and Table 3]. During the 10-year period, fully susceptible MIC was observed for azithromycin consistently, except for 2010 and 2011 [Figure 3 and Table 3], when resistant isolates were observed. MIC as high as 1 µg/mL was reported. From 2012 to 2017, all isolates were found to be susceptible to azithromycin with no MIC creep [Table 3].
| Antibiotics | MICs |
MIC (µg/mL) at the indicated time of isolate recovery |
MIC (µg/mL) at indicated time of isolate recovery |
P value |
|---|---|---|---|---|
| 2008–2012 | 2013–2017 | |||
| Penicillin |
MIC50 MIC90 Geometric mean MIC range |
8 0.5 6 0.064–>32 |
16 4 12 0.19–>32 |
0.06 0.04 0.06 |
| Tetracycline |
MIC50 MIC90 Geometric mean MIC range |
0.5 2 0.25 0.125–32 |
16 2 10 0.094-32 |
0.04 >0.05 <0.05 |
| Ciprofloxacin |
MIC50 MIC90 Geometric mean MIC range |
0.5 2 13 0.002–32 |
4 32 20 0.032–>32 |
0.06 0.04 >0.05 |
| Azithromycin |
MIC50 MIC90 Geometric mean MIC range |
0.125 0.125 0.38 0.016–2 |
0.125 0.25 0.23 0.047–2 |
>0.05 >0.05 >0.05 |
| Ceftriaxone |
MIC50 MIC90 Geometric mean MIC range |
0.002 0.004 0.002 <0.002–0.125 |
0.002 0.012 0.011 <0.002–0.094 |
>0.05 >0.05 >0.05 |
Bold means Statistically significant values. Geometric mean was calculated as per the formula square root square root of X1xX2xX3...Xn, where X is the individual value recorded for MIC
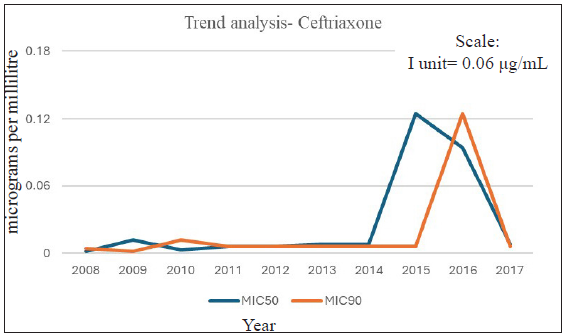
- Ceftriaxone MIC trend analysis (2008–2017).
| Year | Ceftriaxone | Azithromycin | ||||||
|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | % with MIC <0.06 | % with MIC = 0.06-0.25 | MIC50 | MIC90 | % with MIC <1 | % with MIC ≥1 | |
| 2008 | 0.002 | 0.004 | 100 | 0 | 0.064 | 0.125 | 100 | 0 |
| 2009 | 0.012 | 0.002 | 100 | 0 | 0.09 | 0.094 | 100 | 0 |
| 2010 | 0.003 | 0.012 | 100 | 0 | 0.125 | 0.25 | 86.67 | 13.33 |
| 2011 | 0.006 | 0.006 | 92.8 | 7.14 | 0.094 | 0.25 | 85.72 | 14.28 |
| 2012 | 0.006 | 0.006 | 92.8 | 7.14 | 0.19 | 0.5 | 100 | 0 |
| 2013 | 0.008 | 0.006 | 92.8 | 7.14 | 0.008 | 0.25 | 100 | 0 |
| 2014 | 0.008 | 0.006 | 92.8 | 7.14 | 0.008 | 0.09 | 100 | 0 |
| 2015 | 0.125 | 0.006 | 92.8 | 7.14 | 0.125 | 0.32 | 100 | 0 |
| 2016 | 0.094 | 0.125 | 92.8 | 7.14 | 0.094 | 0.125 | 100 | 0 |
| 2017 | 0.008 | 0.006 | 100 | 0 | 0.008 | 0.006 | 100 | 0 |
MIC: Minimum inhibitory concentration (µg/mL). Decreased susceptible for ceftriaxone defined as MIC 0.06-0.25µg/mL
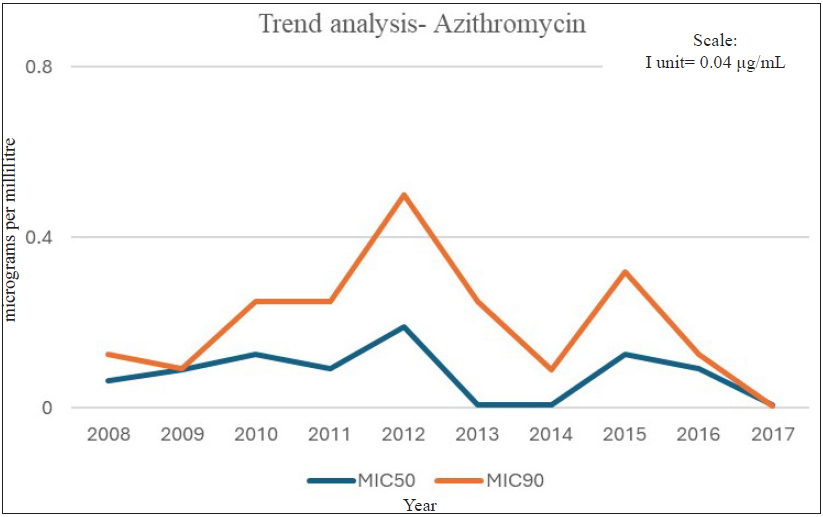
- Azithromycin MIC trend analysis (2008–2017).
A total of 32 isolates were collected from January 2018 to June 2023, though there were none during 2020-2021 (COVID-19 pandemic). Overall, 27 (84.4%) isolates exhibited penicillin resistance (CMR (chromosomally mediated resistance) -28.1%; PPNG-56.2%), and 20 (62.5%) displayed tetracycline resistance (CMR-46.8%; TRNG (tetracycline-resistant Neisseria gonorrhoeae) -15.6%). Quinolone resistance was seen in 30 (93.7%) isolates of which 17 (53.1%) showed HLR. DS to ceftriaxone and cefixime was observed in 3 (9.4%) isolates, and only one (3.1%) demonstrated azithromycin resistance. No isolate displayed concurrent resistance to azithromycin and decreased susceptibility (DS)/ less susceptible (LS) to ceftriaxone/cefixime during this period. All the isolates were susceptible to spectinomycin, and no MDR/XDR-NG was seen.
Discussion
Gonorrhoea is treated empirically based on susceptibility patterns of prevalent isolates rather than testing individual isolates on an emerging basis. The data form the basis for the antibiotics used in the syndromic management of genital discharge in resource-limited settings. This report details the resistance trends to various antimicrobials used for the treatment of gonorrhoea over a ∼15-year surveillance period at a tertiary care hospital in India. In our isolates, chromosomal resistance to penicillin was seen in 16.5% of cases during the 10-year period (2008-2017) and 28.1% thereafter. Previously, a low rate of chromosomal resistance had been reported in India (3.7%).14 Approximately 56% of gonococcal isolates displayed plasmid-mediated resistance to penicillin via beta-lactamase production. Gonococcus harbours TEM-1 type β-lactamases. Mutations in TEM genes contributing to the Extended-spectrum beta-lactamases (ESBL) phenotype have been previously documented in gram-negative bacteria, especially in Enterobacteriaceae. Sequencing of the TEM1 gene in our gonococcal isolates has not demonstrated mutations so far. However, there is a need to be cautious of its evolution.15 Recently plasmid-mediated blaCTXMI resistant gene was obtained in gonococcal isolates endocervical swabs of female patients from Iraq.16 The prevalence of beta-lactamase producing NG varies between 8 and 88.2%, as reported from various parts of India17. Its prevalence rates in Japan, Australia, and the US have been reported as 1.4%, 11.6%, and 42.2%, respectively.18-20 In the current study, no isolate was fully susceptible to penicillin, while 27.8% were less susceptible during a 10-year period and 15.6% thereafter. Further, 31.1% of gonococcal isolates were found to be TRNG, while 30.4% had chromosomal resistance to tetracycline, and a marked decrease in TRNG isolates (15.6%) was seen in the study period 2018-23. However, a study from India showed increasing trends of TRNG from 28.1% to 58.5% between the years 2009 and 2011.17 Over the 10-year period, it is frightening to note that 85.5% of isolates were ciprofloxacin resistant. Adding to our distress is the high percentage of isolates (75.5%) demonstrating HLR even though it was discontinued as first-line therapy in India in the early 2000s. We did observe a decrease in HLR isolates (56.5%) from 2018 onwards. There is wide variation in ciprofloxacin resistance rates in India (49.1% to 100%).16 Similar variation in ciprofloxacin resistance has been reported from different parts of the world: 10.2% to 27.3% in USA, 33.6% in Australia, and 78.2% in Japan.18-21 In the present study, the high percentages of resistance reported towards penicillin, tetracycline, and ciprofloxacin and increasing trends of resistance to these antibiotics reinforce the fact that these antimicrobials should not be used for the treatment of gonorrhoea. Our data is in line with the recently published data from other regions: the Western Pacific Region, Latin America and the Caribbean, Russia, Europe, and Africa.22 It also demonstrates that penicillin, tetracycline, and ciprofloxacin resistance has continued to increase in spite of these antimicrobials not being used for the treatment of gonococcal infections, indicating ongoing selective pressure produced by the use of these antimicrobials to treat other infections. This study further indicates that penicillin, tetracycline, and ciprofloxacin are no longer viable options for future genital discharge treatment algorithms in India.
In resource-limited settings, the higher costs of new antimicrobials, such as ESCs, pose a significant financial burden. Moreover, replacing quinolones with these cephalosporins may only offer a temporary solution. Mathematical models have predicted that we expect it to work for 10 years or more.23 It is not a matter of whether NG will become resistant to ESCs; it is a matter of when it will happen. Therefore, it is important to focus on strategies to prolong the life of cephalosporins.
ESCs are the mainstay of treatment for gonorrhoea. WHO, as per the 2023 update, recommends a single 1 g intramuscular dose of ceftriaxone for adults and adolescents with genital, anorectal, and/or oropharyngeal gonococcal infections. Cefixime 800 mg orally is suggested as an alternative treatment with a test of cure. If a test of cure is unavailable or a pharyngeal infection is suspected, oral cefixime 800 mg plus azithromycin 2 g is advised. For cases involving resistance, allergies, or non-availability of cephalosporins, spectinomycin or gentamicin combined with azithromycin is recommended as an alternative therapy.24 This approach, advocated for by the WHO, is both proactive and pre-emptive and is aimed at limiting the emergence of XDR-NG, particularly in regions where there is limited surveillance in high-risk populations, e.g. MSM and commercial sex workers. As per recent CDC guidelines, the dose of ceftriaxone has been raised from 250 mg to 500 mg (cefixime 800 mg), and if chlamydial infection does not get ruled out, then add doxycycline 100 mg orally 2 times/day for 7 days.25 Ceftriaxone is a bactericidal drug with time-dependent killing. We can predict ‘MIC creep” over time with the help of MIC 50 and MIC 90 data and the same may be used for dosage escalation. Therefore, recent changes in recommended therapy from 250 mg to 500 mg by CDC are based on the MIC data from Gonococcal Isolate Surveillance Project (GISP). In our study, no significant change in the MIC of ceftriaxone and azithromycin have been observed. The introduction of dual therapy by NACO in 2007, comprising of ESCs and azithromycin, was an attempt to retard the evolution of resistance. The combination continues to be efficacious.
Studies from Japan and Korea have reported resistance to penicillin, tetracycline, fluoroquinolones, and other oral cephalosporins in up to 40% of the isolates.26 With quinolone resistance already high, the loss of cephalosporin efficacy would make the control of gonococcal infection even more challenging, especially in resource-limited settings. Spectinomycin remains an effective alternative for treating uncomplicated gonorrhoea. There was only a single report of spectinomycin-resistant NG from India in 2005 and none thereafter.27 We have not observed any resistance to spectinomycin during the study period. Nevertheless, as spectinomycin is administered intramuscularly, it is impractical for resource-constrained settings or where less-skilled healthcare workers are available. Furthermore, spectinomycin is not advised for treating pharyngeal infections. Additionally, NG is known to develop HLR to spectinomycin from a single-step mutation. Widespread usage can lead to the development of resistance quickly, as has been seen in the past.28 The prevalence of azithromycin resistance, although low was observed in 2.6% and thereafter in 3.1% of gonococcal isolates in our study. Although azithromycin has shown >95% clinical effectiveness for treating urethral and endocervical gonococcal infections, it is recommended only as part of dual therapy due to the high risk of developing resistance when macrolides are used as monotherapy for gonorrhoea.29 Monotherapy, even with a higher dose of azithromycin, i.e. 2 g is not endorsed. Resistance has been documented even at this higher dose.30 Further, this dosage is linked to an increase in adverse events e.g. gastrointestinal issues and cardiac effects. Azithromycin is frequently used in the community for treatment of other bacterial infections like pneumonia and recently, enteric fever and has been cited as the most used over-the-counter antibiotic in India.31 This may also be a contributing factor in the emergence of resistance.
A decreased ability to treat NG poses a considerable threat to public health due to its high disease burden, potential for severe sequelae, and increased risk of ongoing transmission facilitated by the prolonged duration of infectiousness. Also, infection with NG has been associated with increased transmission and acquisition of HIV. Gonorrhoea management is further complicated by its ability to acquire resistance genes from another non-pathogenic Neisseria spp. Due to the rapid development of gonococcal drug resistance, strategies for antimicrobial stewardship are required on priority. This includes the adoption of standardised, rational, and controlled prescription practices for treating genital discharge syndrome to curb resistance. It is important to remember that antibiotics are a shared resource, and their misuse not only impacts the health of the individual but also society at large. Research initiatives focused on the development of novel molecules with unique modes of action should be encouraged to increase the antibiotic pipeline. Phase III clinical trials of new anti-gonococcal antibiotics, zoliflodacin, solithromycin, and gepotidacin have shown encouraging results for this WHO ‘high” priority pathogen, and perhaps one of them could be a solution.32-35
Limitations
The main limitation of our study is the smaller number of isolates and that these strains were collected from a single site in India. Additionally, demographic, behavioural, or clinical data has not been included. This could have provided a better understanding of the disease epidemiology, an issue that has also been highlighted in the enhanced gonococcal antimicrobial surveillance programme (EGASP). Further, in this study, we have analysed isolates obtained from an STI clinic only, data from the community are lacking, and so are data from extragenital sites. Lastly, there is a paucity of data from 2020 to 2021 due to the COVID-19 pandemic.
Conclusion
Our study highlights that the resistance rates to azithromycin are still below the WHO-designated cut-off in India and there is no HLR to azithromycin. In addition, we have noted that the clinical isolates of NG continue to be largely susceptible to the ESCs (ceftriaxone & cefixime). However, MIC -based monitoring of crucial antibiotics (azithromycin and ESCs) is vital to identify the emergence of any new resistance. Simultaneously, there is an urgent need to develop rapid genetic testing methodologies for AMR. A personalised treatment strategy will promote the judicious utilisation of antimicrobials, including the conservation of the last-line antimicrobials, thereby enhancing public health efforts to control both gonorrhoea and AMR. Furthermore, it is essential to allocate resources towards strengthening local surveillance for a deeper understanding of transmission dynamics and enabling informed control strategies. These would involve designing and implementing activities aimed at improving the evaluation of treatment outcomes and extragenital (pharyngeal) infections at community and regional healthcare levels. Also, there should be ongoing antimicrobial surveys, including test-of-cure assessments, conducted among key populations at all healthcare levels.
Ethical approval
The research/study was approved by the Institutional Review Board at Institute Ethics Committee, AIIMS, New Delhi, number IEC/NP-A-52/2006, IEC/OP-18/2013, IEC/RP-01/05.08.2013, IESC/T-429/28.11.14, dated 26.06.2013, 19.06.2013, 05.08.2013, 28.11.2014.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The study was funded by grant no IRIS-ID No.2006-0685E, Indian Council of Medical Research, Department of Health Research, Ministry of Health & Family Welfare, grant no. BT/PR 12530/MED/32/385/2015, Department of Biotechnology, Government of India and Department of Microbiology, AIIMS, New Delhi.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74:S12-6.
- [PubMed] [Google Scholar]
- Accountability for the global health sector strategies 2016–2021: actions for impact. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO
- Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: Challenges and opportunities. Sex Transm Infect. 2013;89:iv36-41.
- [CrossRef] [PubMed] [Google Scholar]
- The European gonococcal antimicrobial surveillance programme (Euro-GASP)--a sentinel approach in the European Union (EU)/European Economic Area (EEA) Sex Transm Infect. 2013;89:iv16-8.
- [CrossRef] [PubMed] [Google Scholar]
- Is the tide turning again for cephalosporin resistance in Neisseria gonorrhoeae in europe? Results from the 2013 European surveillance. BMC Infect Dis. 2015;15:321.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cephalosporin resistance in Neisseria gonorrhoeae. J Glob Infect Dis. 2010;2:284-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gonorrhoea treatment combined with population-level general cephalosporin and quinolone consumption may select for Neisseria gonorrhoeae antimicrobial resistance at the levels of NG-MAST genogroup: An ecological study in europe. J Glob Antimicrob Resist. 2020;23:377-84.
- [CrossRef] [PubMed] [Google Scholar]
- World health organization global gonococcal antimicrobial surveillance program (WHO GASP): Review of new data and evidence to inform international collaborative actions and research efforts. Sex Health. 2019;16:412-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis. 2018;24:735-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Changing trends of antimicrobial susceptibility patterns of Neisseria gonorrhoeae in India and the emergence of ceftriaxone less susceptible N. gonorrhoeae strains. J Antimicrob Chemother. 2007;60:582-6.
- [CrossRef] [PubMed] [Google Scholar]
- Emergence of decreased susceptibility to extended-spectrum cephalosporins in Neisseria gonorrhoeae in India. Natl Med J India. 2013;26:26-8.
- [PubMed] [Google Scholar]
- Emergence of quinolone resistance and cephalosporin MIC creep in Neisseria gonorrhoeae isolates from a cohort of young men in Kisumu, Kenya, 2002 to 2009. Antimicrob Agents Chemother. 2011;55:3882-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antibiotic susceptibility testing by the CDS method. A manual for medicine and veterinary laboratories 2016; South Eastern Area Laboratory Services, 8th edition, 2011; p. 45-9.
- Antimicrobial susceptibility profile of resistance phenotypes of Neisseria gonorrheae in India. Sex Transm Dis. 2008;35:588-91.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular characterization of decreased susceptibility to ceftriaxone and genotyping of Neisseria gonorrheae isolates in New Delhi, India. Diagn Microbiol Infect Dis. 2021;101:115423.
- [CrossRef] [PubMed] [Google Scholar]
- Occurrence Of CTX-M I gene in Beta lactam resistance Neisseria gonorrhoeae isolated form women with endocervical infection. J Popl Ther Clin Pharmacol. 2023;30:49-53.
- [Google Scholar]
- Monitoring antimicrobial resistance in Neisseria gonorrhoeae in selected countries of the WHO South-East Asia region between 2009 and 2012: A retrospective analysis. Sex Transm Infect. 2013;89:iv28-35.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of TEM-135 beta-lactamase in penicillinase-producing Neisseria gonorrhoeae strains in Japan. Antimicrob Agents Chemother. 2010;54:3021-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neisseria gonorrhoeae antimicrobial susceptibility surveillance – The gonococcal isolate surveillance project, 27 sites, United States, 2014. MMWR Surveill Summ. 2016;65:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Australian gonococcal surveillance programme, 1 July to 30 September 2015. Commun Dis Intell Q Rep. 2016;40:E179-81.
- [PubMed] [Google Scholar]
- Antimicrobial resistance and molecular typing of Neisseria gonorrhoeae isolates in Kyoto and Osaka, Japan, 2010 to 2012: Intensified surveillance after identification of the first strain (H041) with high-level ceftriaxone resistance. Antimicrob Agents Chemother. 2013;57:5225-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017;14:e1002344.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Projecting the development of antimicrobial resistance in Neisseria gonorrhoeae from antimicrobial surveillance data: A mathematical modelling study. BMC Infect Dis. 2023;23:252.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Updated recommendations for the treatment of Neisseria gonorrhoeae, Chlamydia trachomatis and Treponema pallidum (syphilis), and new recommendations on syphilis testing and partner services. Geneva: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO
- Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
- [PubMed] [PubMed Central] [Google Scholar]
- Trends of sexually transmitted diseases and antimicrobial resistance in Neisseria gonorrhoeae. Int J Antimicrob Agents. 2008;31:S35-9.
- [CrossRef] [PubMed] [Google Scholar]
- First case of spectinomycin resistant Neisseria gonorrhoeae isolate in New Delhi, India. Sex Transm Infect. 2005;81:186-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of spectinomycin use on the prevalence of spectinomycin-resistant and of penicillinase-producing Neisseria gonorrhoeae. N Engl J Med. 1987;317:272-8.
- [CrossRef] [PubMed] [Google Scholar]
- Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex Transm Infect. 2010;86:422-6.
- [CrossRef] [PubMed] [Google Scholar]
- Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011) Sex Transm Dis. 2012;39:877-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Consumption of systemic antibiotics in India in 2019. Lancet Reg Health Southeast Asia. 2022;22:100025.
- [CrossRef] [Google Scholar]
- Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. Geneva: World Health Organization; 2017. (WHO/EMP/IAU/2017.12). Available from: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 Last accesed on 2024 July 25
- GARDP, Innoviva Specialty Therapeutics. Positive results announced in largest pivotal phase 3 trial of a first-in-class oral antibiotic to treat uncomplicated gonorrhoea. Available from: https://gardp.org/positive-results-announced-in-largest-pivotal-phase-3-trial-of-a-first-in-class-oral-antibiotic-to-treat-uncomplicated-gonorrhoea/ Last accessed on 2024 July 25.
- Phase 3 trial of treating gonorrhoea with solithromycin. Lancet Infect Dis. 2019;19:928.
- [CrossRef] [PubMed] [Google Scholar]
- GSK. GSK announces positive headline results from EAGLE-1 phase III trial for gepotidacin in uncomplicated urogenital gonorrhoea (GC). Available from: https://www.gsk.com/en-gb/media/press-releases/eagle-1-phase-iii-data-show-potential-for-gepotidacin-as-a-new-oral-treatment-option-for-uncomplicated-gc/ Last accessed 2025 Jan 10.






