Translate this page into:
Anaplastic lymphoma kinase-positive primary cutaneous anaplastic large cell lymphoma- Is it a new variant?
2 Department of Dermatology, St. John's Medical College Hospital, Bangalore, Karnataka, India
3 Department of Pathology, St. John's Medical College Hospital, Bangalore, Karnataka, India
Correspondence Address:
Muthu Sendhil Kumaran
Department of Dermatology, Venerology and Leprology, PGIMER, Chandigarh
India
| How to cite this article: Kumaran MS, Jithendriya M, Nagaraj P, Tirumalae R, Jayaseelan E. Anaplastic lymphoma kinase-positive primary cutaneous anaplastic large cell lymphoma- Is it a new variant?. Indian J Dermatol Venereol Leprol 2012;78:354-357 |
Abstract
Anaplastic large cell cutaneous lymphomas are clinically and pathologically heterogeneous, CD30 + (Ki-1) lymphoproliferative disorders. The importance of anaplastic lymphoma kinase (ALK) positivity is well known in the prognosis of primary systemic anaplastic large cell cutaneous lymphomas; however, the same in primary cutaneous anaplastic large cell cutaneous lymphomas is not much clear. Herein we report a 65-year-old male with an 18-month history of minimally pruritic localized nodulo-plaque lesion over lower back. Histology revealed cutaneous large cell lymphoma and immunohistochemical staining showed positivity for CD30, CD3 and ALK. The role of ALK positivity in pcALCL is discussed in this article.Introduction
Cutaneous CD30 + anaplastic large cell lymphomas (ALCLs) are clinically and pathologically heterogeneous, leading to difficulty in its diagnosis and classification. Expression of CD30 (an activation marker for B or T cells) in more than 75% of neoplastic cells characterizes this group of lymphoproliferative disorders. [1],[2]
With the use of molecular and clinical criteria, three entities of ALCL have been identified: Primary systemic anaplastic lymphoma kinase (ALK)-positive ALCL, primary systemic ALK-negative ALCL and primary cutaneous ALCL (pcALCL). Systemic ALCL is clinically distinct from cutaneous ALCL, though they share several morphological features. It is important to make this distinction as their prognosis is also different. Primary cutaneous CD30 + ALCL is ALK-positive only in extremely rare instances. [3] There is a paucity of reports on ALK-positive pcALCL.
Case Report
A 65-year-old male presented with an 18-month history of pruritic localized nodulo-plaque lesion over lower back. He was applying clobetasol propionate ointment on and off over the lesion with partial relief. He was otherwise asymptomatic. His personal and family history was non contributory. There was no history of preceding insect bite or skin lesions.
Clinical examination revealed 4 × 6 cms solitary, erythematous, infiltrated plaque studded with many nodules and ulcers with crusting [Figure - 1]. No enlarged lymphnodes or any other significant skin lesions were seen. Systemic examination was normal.
 |
| Figure 1: Solitary, erythematous, infiltrated plaque studded with many nodules, with focal areas of ulceration |
Histology showed, epidermal ulceration, dermis showing sheets of atypical lymphoid cells with pleomorphic, hyperchromatic nuclei. A few mononuclear cells showed embryonic nuclei admixed with neutrophils and lymphocytes [Figure - 2]. Areas of necrosis were also seen. Immunohistochemical staining showed positivity for CD30, CD3 and ALK [Figure - 3], [Figure - 4] and [Figure - 5].
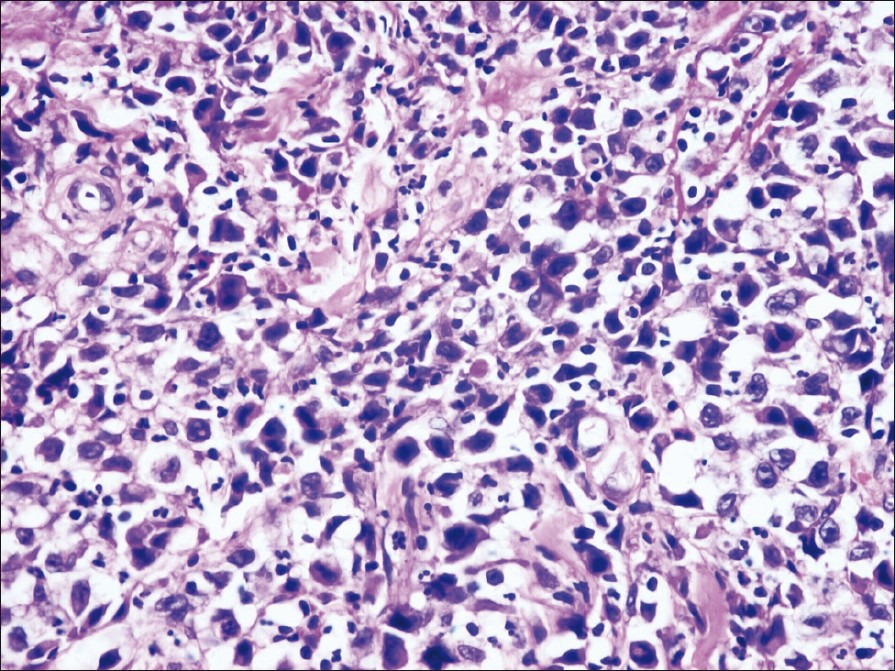 |
| Figure 2: Histopathology of the nodules showing a few mononuclear cells with embryonic nuclei admixed with neutrophils and lymphocytes (H and E, ×400) |
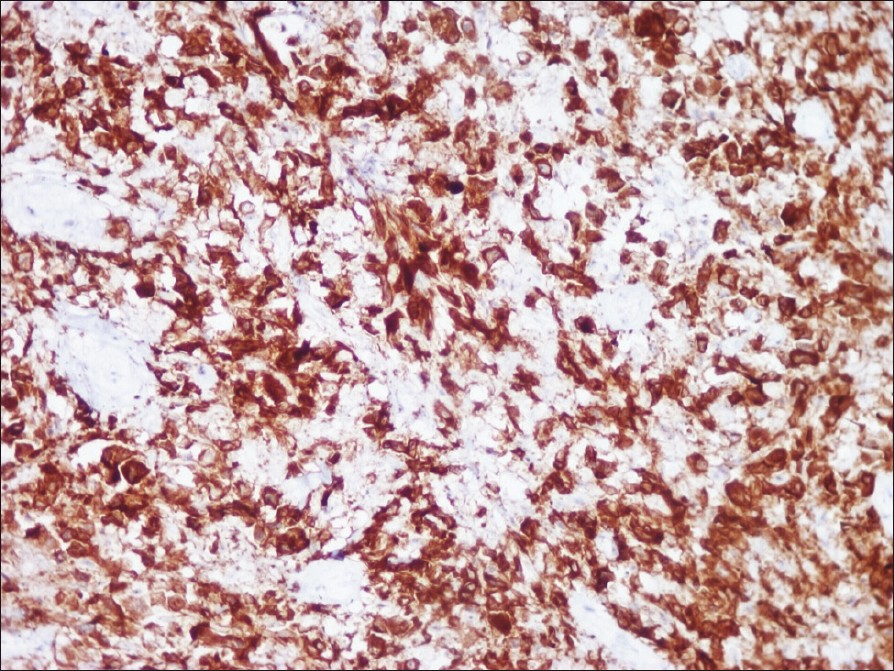 |
| Figure 3: Immunohistochemical staining showing diffuse positive staining of anaplastic cells (CD 30, ×400) |
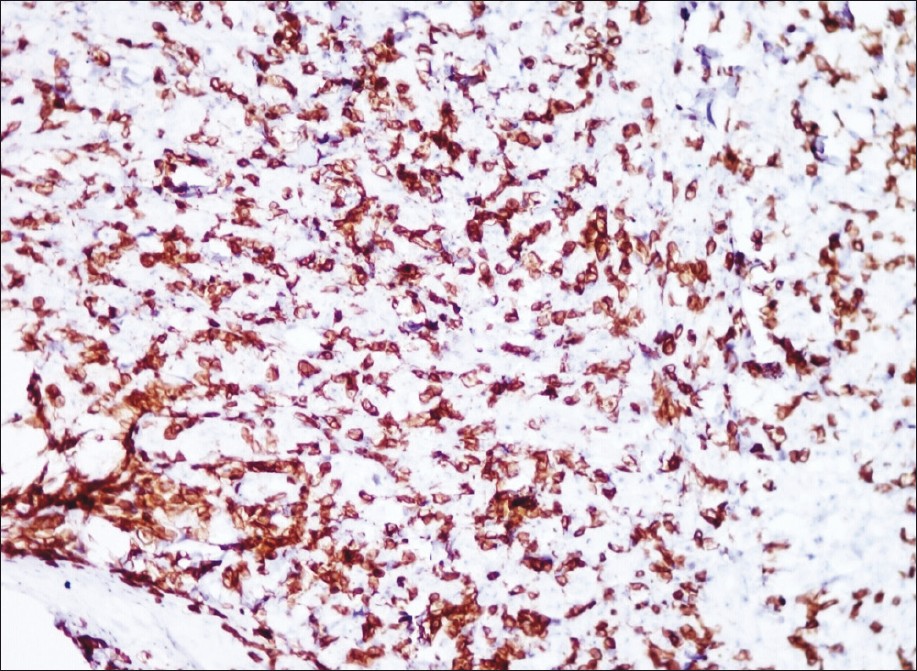 |
| Figure 4: Immunohistochemical staining showing diffuse positive staining of lesional cells (CD 3, ×400) |
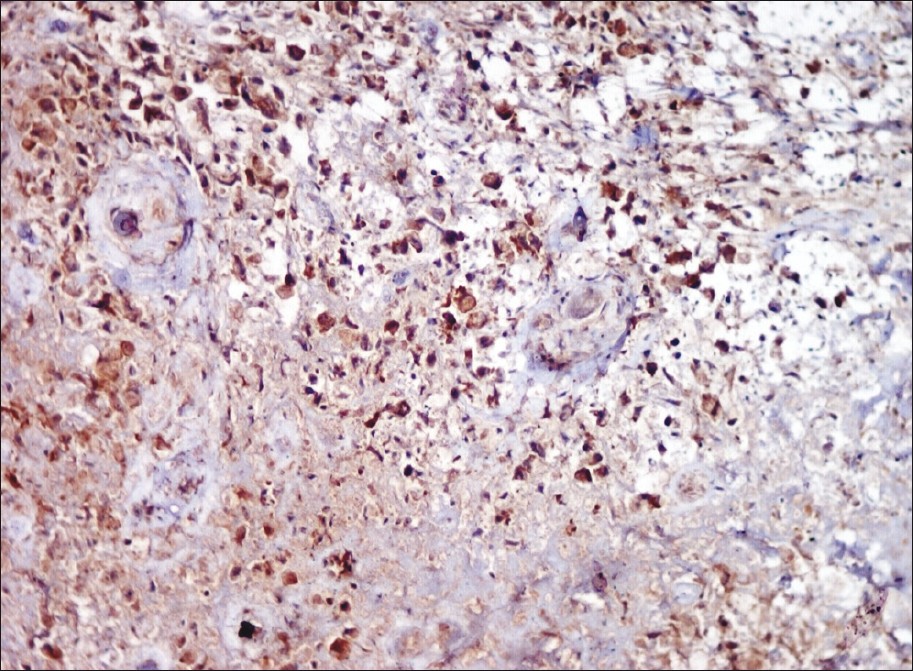 |
| Figure 5: Immunohistochemical staining showing diffuse positive staining of lesional cells (ALK, ×400) |
With the above findings the patient was subjected to thorough investigations in the form of regular hemogram, peripheral blood smear, bone marrow biopsy, CT scan of chest and abdomen, which were all found to be normal. Hence, with above findings and as per the criteria suggested by Beljaards et al., we made a diagnosis of pcALCL confined only to skin and was staged as 1b as per EORTC staging.
Discussion
ALCL initially described in 1985, is second most common type of cutaneous T-cell lymphoma. [4] It constitutes 9% of all cutaneous lymphomas. [4] pcALCLs may be primary (de novo) or secondary which arises from anaplastic transformation of another lymphoma such as mycosis fungoides. Extranodal ALCL has been found in the skin (21%). [5]
pcALCL shows a higher expression of CCR10 and CCR8, chemokines that direct cells to the skin. This explains why pcALCL has such a low rate of extracutaneous dissemination. [6] In a subset of ALCLs translocation at the t (2;5) chromosome is observed which is associated with the fusion of the nucleophosmin (NPM) gene at 5q35 with the receptor tyrosine kinase ALK gene at 2p23 encoding the NPM-ALK fusion protein. [7],[8] The updated World Health Organization (WHO) classification of hematopoietic tumors and lymphoid tissues recognizes this subclassification, dividing systemic ALCL into ALK-positive and ALK-negative disease. [5]
pcALCL usually presents typically as solitary reddish brown ulcerating nodules or tumors or indurated plaques. Generalized or multifocal lesions are seen in about 20% of the patients. [9] Lymphadenopathy may be present as involvement of the draining regional lymph nodes occuring in approximately 25% of patients. Our case had an indolent infiltrated erythematous plaque studded with many nodules with ulceration. There were no enlarged draining lymph nodes or any systemic manifestation. According to ISCL-EORTC TNM [10] classification our index case is classified as T1b stage.
Histopathology is of paramount importance in diagnosing this condition; pcALCL consists of diffuse nonepidermotropic infiltrates of cohesive sheets of large CD30 + tumor cells. It shows large lymphoid cells also known as "hallmark cells", with embryonic nuclei, multiple prominent nucleoli and abundant cytoplasm. A significant eosinophilic infiltrate in histology may be an indicator for lymph node involvement. [11] In secondary ALCL, which has evolved from pre-existing mycosis fungoides, large hyperchromatic cerebriform cells may be seen. [9] In our patient histology revealed, ulceration of epidermis, sheets of lymphoid cells with atypical, hyperchromatic nuclei in dermis. A few mononuclear cells showed characteristic embryonic nuclei. Immunohistochemistry showed positivity for CD3, CD30 and ALK.
In view of the ALK expression, it is imperative to exclude systemic disease. CT scans of the chest, abdomen and pelvis must be performed for staging and differentiating the primary cutaneous form from the systemic form involving the skin. The latter has systemic lymphadenopathy other than regional lymphadenopathy associated with skin lesions. In our patient CT of chest, abdomen and pelvis did not show any systemic involvement. Systemic ALCL can initially present as isolated cutaneous disease even if no extracutaneous disease is found at presentation; a close follow-up is mandatory in this case, in view of ALK being positive.
Site of origin and ALK expression are important prognostic markers. The 5-year survival rate is typically around 90% for pcALCL. [12] In fact, cases of pcALCL may regress without any therapeutic intervention. [9] Newer data suggest that the overall 5-year survival in pcALCL are affected by the extent of limb involvement. For patients without extensive limb disease, 5-year survival remains around 90%, with extensive limb involvement it reduces to 50%, is also associated with more rapid disease progression and decreased response to treatment. This cannot be generalized to our patient as the lesion was localized to back.
Shiota first observed the impact of NPM-ALK expression on patient outcome in 1995. [13] Patients with expression of ALK have a 5-year survival rate ranging from 70 to 80%. In a series of 57 cases of adult ALCL, including T-cell and null cell phenotypes, 5-year overall survival (OS) was 67%. [13]
There have been conflicting reports with regards to the expression of NPM-ALK in ALCL. De coteau et al., compared the expression of ALK in pcALCL and nodal ALCL and found that t (2; 5) translocation was not seen in pcALCL, but was associated with 66% of nodal ALCL. [14] In contrast, Beylot - Barry et al., [3] reported the presence of t (2; 5) translocation even in pcALCL and attributed it the use of better techniques like nested PCR. They noted that two patients had many cutaneous lesions and three also had extracutaneous lesions. The exact percentage of pcALCL with ALK-positive and its importance in prognosis in pcALCL is still unavailable. Although very few cases of pcALCL with ALK-positive been reported, its prognosis and classification is yet to be resolved. Hence, follow-up of these cases may solve this uncertainty as to include pcALCL as a part of ALK-positive systemic lymphoma group or to consider it as a distinct entity.
pcALCL is a rare condition, the diagnosis of which rests on good clinicopathological correlation coupled with early biopsy. Immunostains are a must to clinch the diagnosis. The disease should be distinguished from the more common systemic ALCL, as it portends a vastly better prognosis. The significance of ALK expression in pcALCL is yet to be unraveled, encouraging us to report this rare case.
| 1. |
Diamantidis MD, Papadopoulos A, Kaiafa G, Ntaios G, Karayannopoulou G, Kostopoulos I, et al. Differential diagnosis and treatment of primary, cutaneous, anaplastic large cell lymphoma: Not always an easy task. Int J Hematol 2009;90:226-9.
[Google Scholar]
|
| 2. |
Savage KJ, Lee-Harris N, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK-negative anaplastic large-cell lymphoma (ALCL) is clinically and immunophenotypically different from both ALK-positive ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496-504.
[Google Scholar]
|
| 3. |
Beylot-Barry M, Lamant L, Vergier B, DeMuret A, Fraitag S, DeLord B, et al. Detection of t (2;5) (p23q35) translocation of reverse transcriptase polymerase chain reaction and in situ hybridization in CD-30 positive primary cutaneous lymphoma and lymphamatoid papulosis. Am J Pathol 1996;149:483-92.
[Google Scholar]
|
| 4. |
Querfeld C, Khan I, Mahon B, Nelson BP, Rosen ST. Primary Cutaneous and Systemic Anaplastic Large Cell Lymphoma. Oncology 2010;24: 1-22.
[Google Scholar]
|
| 5. |
Delsol G, Ralfkiaer E, Stein H, Wright D, Jaffe ES. Anaplastic large cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Tumours of Haematopoietic and Lymphoid Tissues. Pathology and Genetics. World Health Organization Clasification of Tumours. Lyon, France: IARC Press; 2001. p. 230-5.
[Google Scholar]
|
| 6. |
Van Kester MS, Tensen CP, Vermeer MH, Dijkman R, Mulder AA, Szuhai K, et al. Cutaneous anaplastic large cell lymphoma and peripheral T-cell lymphoma NOS show distinct chromosomal alterations and differential expression of chemokine receptors and apoptosis regulators. J Invest Dermatol 2010;130:563-75.
[Google Scholar]
|
| 7. |
Kinney MC, Kadin ME. The pathologic and clinical spectrum of anaplastic large cell lymphoma and correlation with ALK gene dysregulation. Am J Clin Pathol 1999;111: S56-67.
[Google Scholar]
|
| 8. |
Mahalingam M, Bhawan J. Cutaneous anaplastic large cell lymphoma: A case report. Indian J Dermatol Venereol Leprol 2004;70:168-71.
[Google Scholar]
|
| 9. |
Stein H, Foss HD, Durkop H, Marafioti T, Delsol G, Pulford K, et al. CD30+ anaplastic large cell lymphoma: A review of its histopathologic, genetic and clinical features. Blood 2000;96:3681-95.
[Google Scholar]
|
| 10. |
Jaffe ES. The 2008 WHO classification of lymphomas: Implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program 2009:523-31.
[Google Scholar]
|
| 11. |
Beljaards RC, Kaudewitz P, Berti E, Gianotti R, Neumann C, Rosso R, et al. Primary cutaneous CD30+ large cell lymphoma: Definition of a new type of cutaneous lymphoma with a favorable prognosis. Cancer 1993;71:2097-104.
[Google Scholar]
|
| 12. |
Woo DK, Jones CR, Vanoli-Story MN, Kohler S, Reddy S, Advani R, et al. Prognostic factors in primary cutaneous anaplastic large cell lymphoma: Characteristic status of clinical subset with worse outcome. Arch Dermatol 2009;145:667-74.
[Google Scholar]
|
| 13. |
Shiota M, Nakamura S, Ichinohasama R, Abe M, Akagi T, Takeshita M, et al. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: A distinct clinicopathologic entity. Blood 1995;86:1954-60.
[Google Scholar]
|
| 14. |
De Coteau J, Butmarc JR, Kinney M, Kadin M. The t (2; 5) chromosomal translocation is not a common feature of primary cutaneous CD 30+ lymphoproliferative disorders: Comparison with anaplastic large cell lymphoma of nodal origin. Blood 1996;87:3437-41.
[Google Scholar]
|
Fulltext Views
1,906
PDF downloads
2,477





