Translate this page into:
Androgenetic alopecia: An update
2 Department of Pathology, College of Medicine, King Faisal University, Kingdom of Saudi Arabia,
3 Department of Plastic Surgery, Amrita Institute of Medical Sciences and Research Centre, Kochi, Kerala, India,
Correspondence Address:
Feroze Kaliyadan
Department of Dermatology, King Faisal University Hofuf, Saudi Arabia
| How to cite this article: Kaliyadan F, Nambiar A, Vijayaraghavan S. Androgenetic alopecia: An update. Indian J Dermatol Venereol Leprol 2013;79:613-625 |
Abstract
Androgenetic alopecia (AGA) is one of the commonest reasons for dermatological consultation. Over the last few years our understanding of the pathophysiology of AGA has improved and this has paved way for better diagnostic and therapeutic options. Recent research has dwelled on the role of stem cells in the pathophysiology of AGA and has also identified newer genetic basis for the condition. Dermoscopy/trichoscopy has emerged as a useful diagnostic tool for AGA. While the major treatment options continue to be topical minoxidil, systemic Finasteride and hair transplantations, newer modalities are under investigation. Specific diagnostic and treatment recommendations have also been developed on evidence based principles. This article reviews the recent concepts in relation to AGA. With regards to the pathophysiology we have tried to stress on recent knowledge of the molecular and genetic basis of AGA. We have emphasized on an evidence based approach for treatment and diagnosis.Introduction
"What (Time) hath scanted men in hair he hath given them in wit" - Shakespeare (from "The comedy of errors")
During the times of Shakespeare, when the pathogenesis of androgenetic alopecia (AGA) was not clearly understood, the only option for those affected was to live with it, other than using wigs for cosmetic purposes. Now, we have a deeper understanding of the genetics, molecular basis and pathophysiology of AGA, which has paved the way for effective treatment modalities. Alopecia induced by androgens in genetically predisposed individuals is termed androgenetic alopecia. [1] The importance of understanding and treating this condition is growing because of the emphasis that modern society lays on ′looking good′. This article attempts to review the topic of AGA with emphasis on recent advances, especially with regard to genetics, pathophysiology and treatment.
Psychological effects of AGA
Androgenetic alopecia (AGA) has always been recognized as having significant psychological effects on affected patients. Vincent van Gogh′s famous painting ′On the threshold of eternity′ is thought to depict deep depression, partly attributed to a balding scalp. [2] One of the most important psychological aspects related to AGA is related to the true or imagined perceptions of others. [3] Various studies have demonstrated that AGA can have a significant negative impact on the quality of life of the affected persons. [1] A study by Kranz [4] of 160 university students with AGA revealed that the psychological distress due to AGA was not dependent on the age of the patient or stage of baldness. For women affected with AGA the main factors contributing to psychological distress were - inability to style their hair, dissatisfaction with their appearance, concern about the continuing hair loss and concern about others noticing their hair loss. [5]
Epidemiology
Men
Androgenetic alopecia (AGA) is considered to be the most common type of baldness characterized by progressive hair loss. [6] AGA can affect all races, but the prevalence rates vary. Prevalence is considered to be highest in Caucasians. It is estimated that prevalence rates in Caucasian populations is around 30% for men in their 30s, 40% for men in their 40s and 50% for men in their 50s. [6] In the Indian context, a population based study of 1005 subjects showed a 58% prevalence of AGA in males aged 30-50 years. [7] In oriental races, a lower prevalence has been shown. In a Chinese study by Wang et al., [6] the overall prevalence was 21.3%, while in a Korean study, the overall prevalence was 14.1%. [8] All studies demonstrate a gradual increase in incidence with age.
Regarding the commonest type/grade of presentation (according to the Norwood classification), studies have shown differing results. A large study in the Indian population had type II as the commonest presentation of AGA. [9] Another study in an Indian population had type II and III as the commonest presentation. [1] The Chinese study by Wang et al., [6] had type IV as the commonest type, while the Korean study by Paik et al., had type III as the commonest type. [8]
Women
Epidemiological studies of AGA in women are fewer in number. A study by Norwood, [10] showed a total prevalence of around 19% in a population of 1006 Caucasian patients. In a Chinese population study, the prevalence was only 6.0% and a Korean study had a relatively similar lower prevalence of 5.6%, suggesting that like in men, the prevalence is considered to be lower in oriental races compared to Caucasians. [6],[8] The incidence of AGA in women also tends to increase with age. [10],[11] It should be noted that experts have suggested that female androgenetic alopecia is not exactly the female counterpart of male AGA. A better term for female AGA would be ′female pattern alopecia′ or ′female pattern hair loss′. The clear difference in the clinical pattern of male and female pattern hair loss suggest that these are two separate entities. This is also based on studies which have shown no clear relation between excess testosterone levels and female pattern hair loss. [10]
Pathogenesis
What is AGA all about?
Androgenetic alopecia (AGA) is characterized by stepwise miniaturization of the hair follicle, resulting from alteration in the hair cycle dynamics, leading to vellus transformation of terminal hair follicle. The normal hair cycle has an active growth phase (anagen) which can last from two years to six to seven years. This is followed by a brief stage of regression (catagen) which lasts one to two weeks and then a resting phase (telogen) lasting from five to six weeks to about 100 days. The catagen phase is a process of involution, where a burst of apoptosis occurs in a majority of follicular keratinocytes along with termination of pigment production and condensation of dermal papillae. The result is an upward movement of dermal papillae. In the telogen phase the hair shaft matures into a club (vellus) hair. The hair is eventually shed as a result of combing and washing and the anagen phase begins again.
In AGA, the duration of anagen phase gradually decreases and that of telogen phase increases. As the duration of anagen phase determines the hair length, the maximum length of the new anagen hair becomes shorter than that of its predecessor, leading to miniaturization and eventually a bald appearance. [12],[13]
How did this concept evolve?
The understanding of the hair follicle biology over the last 20 years established the fundamental role of the mesenchyme derived dermal papillae in the maintenance of the hair growth, with the multipotent epithelial stem cells at the bulge giving rise to proliferation and differentiation. [14],[15],[16],[17] Other autocrine, paracrine factors and signaling pathways are also involved in this cross-talk between the dermal papillae and the hair follicle stem cells. [16],[18],[19]
Testosterone and other weaker androgens such as dehydroepiandrosterone and androstenedione are metabolized in many skin tissues. Testosterone can freely penetrate the cell membrane and is converted in the cytoplasm to dihydrotestosterone (DHT) by 5 α reductase (mainly Type II). The DHT strongly binds to androgen receptor (AR) and this complex is translocated to the nucleus, helped by the AR co activators. This results in target gene transcription and finally translation into genes which exert biological activity. [20],[21],[22]
The cross-talk between the dermal papillae and the hair follicle cells which unfolds under the influence of androgens result from the secretion of many factors from the dermal papillae. These have autocrine effect on the dermal papillae itself and paracrine effect on the hair follicle epithelial cells. [23] These factors include growth factors like Insulin like growth factor (IGF-1), basic fibroblast factor (bFGF), vascular endothelial growth factor (VEGF); and cytokines like transforming growth factor beta 1 (TGFβ 1), interleukin 1 alpha (IL -1α) and tumor necrosis factor alpha (TNF α). [24],[25],[26]
Why do people go bald in AGA?
Many of the studies have accepted the role of androgens and the interplay between the dermal papillae and hair follicle as the critical processes involved in miniaturization of hair follicles.
The concentration of DHT along with 5 α reductase and androgen receptors are increased in the balding scalp. [27],[28] The other enzymes involved in conversion of weak androgens to potent androgens are 3 β hydroxysteroid dehydrogenase (3 β HSD) and 17 β hydroxysteroid dehydrogenase (17 β HSD) also show increased activity in AGA. [29] The higher the concentration of androgen and androgen receptor, more the effect on expression of genes which control follicular cycling.
The signaling which follows at the dermal papillae and hair follicle interface in balding person results in premature termination of anagen associated with premature entry into catagen. Catagen occurs as a consequence of decreased expression of anagen maintaining factors, such as the growth factors- IGF-1, bFGF and VEGF. Also, an increased expression of cytokines (TGFβ 1, IL -1α and TNF α) promotes apoptosis. [30] Recently, DKK-1 is found to be up regulated gene by DHT, resulting in inhibition of outer root sheath cells and triggering apoptosis. [31]
Another recent advance is identification of the critical role of Wnt/β catenin signaling pathway in the maintenance of the DPC inductive properties required for hair follicle regeneration and growth of the hair shaft. [32] The androgens and ligand activated AR can negatively influence the Wnt/β catenin signaling pathway. [33] The androgens hamper the pathway by increasing the glycogen synthase kinase (GSK 3β) expression. [34]
The exact roles and processes related to hair follicle stems cells in AGA are not clear. It is considered that while KRT15(hi) stem cells are maintained in bald scalp, there is a defect in conversion of hair follicle stem cells to CD200-rich and CD34-positive progenitor cells, both of which are needed to maintain proper follicular activity. [35]
When do people go bald?
Androgenetic alopecia (AGA) is a multifactorial disorder caused by interactions between several genes and environmental factors.
Genes involved in AGA: A polygenic mode of inheritance is established due to the high prevalence and the wide range of expressed phenotypes in AGA. The genes influence predisposition through DNA sequence variations- single nucleotide polymorphisms, microsatellite repeats, insertion mutations, deletion mutations and copy number variations; or epigenetic modifications such as X chromosome inactivation, hypermethylation (switch off gene expression) or hypomethylation (switch on gene expression) of DNA in gene promoter regions. [36],[37]
The two major genetic risk loci are the X chromosome AR/EDA2R locus and the PAX1/FOX A2 locus on chromosome 20. Recent studies indicate HAD C9 locus on chromosome 7 as a new susceptibility locus. [38],[39],[40],[41]
The androgen receptor gene: The androgen receptor determines the sensitivity of cells to androgen. The AR gene regulates the potency of androgen available to the hair follicle. Of the many AR gene polymorphisms known, the Stu 1 polymorphism has the most significant association with AGA. [42]
Several other genes where associations could not be proved conclusively include 5α reductase, aromatase, estrogen receptor α and IGF-2 genes. The role of Y chromosome needs further comprehensive examination of the genome. [43]
Hair follicle inflammation and environmental factors: The implication of follicular inflammation has been brought out by several studies. The process is slow, subtle and indolent unlike the inflammatory and destructive process in the classical scarring alopecia. Microbial toxins related to Propionibacterium sp., Staphylococcus sp., Malassezia sp., or Demodex could be involved in generation of inflammatory response. [44] Alternatively, keratinocytes may respond to chemical stress from irritants in cosmetics and grooming agents, pollutants and actinic damage as in UV irradiation by producing radical oxygen species and nitric oxide. [45]
Clinical Features and Grading
While there are different grading systems available for AGA, the most accepted is the modified Norwood-Hamilton classification [Table - 1], modified from the earlier Hamilton classification, consisting of seven broad groups and four specific variant types. [1],[46],[47]

In women, typically three patterns have been described: [48]
- Diffuse thinning of the crown area with preservation of the frontal hair line
- Thinning and widening of the central part of the scalp with breach of the frontal hair line
- Thinning associated with bi-temporal recession.
The commonest grading scales used for female androgenetic alopecia are the three-point Ludwig scale [49] and the five-point Sinclair scale [50] [Table - 2] and [Table - 3].


A newer, systematic and universal classification has been suggested by Lee, et al., known as the basic and specific classification (BASP). The basic (BA) types represent the shape of the anterior hairline, and the specific types (SP) represent the density of hair on distinct areas (frontal and vertex). There are four basic types (L, M, C, and U) and two specific types (F and V). The final type is decided by the combination of the assigned basic and specific types. The basic types are classified by the English alphabetical letter shape of the anterior hairline, except L type, which means linear.
Type L - No recession is observed along the anterior border in the frontotemporal region. It appears a linear.
Type M - Recession in the frontotemporal hairline is more prominent than the mid-anterior hairline. The hairline resembles the letter M.
Type C - Recession in the mid-anterior hairline is more prominent than the frontotemporal hairline.
The entire anterior hairline regresses posteriorly in the shape of half-circle, resembling the letter C.
Type U - The anterior hairline recedes posteriorly beyond the vertex forming a horseshoe shape, resembling the letter U.
Type F - This represents a general decrease in the density of hair over the entire scalp, regardless of the anterior hairline. It is usually more marked over the frontal area of the scalp
Type V - Hair loss is seen more distinctly in the vertex than in the frontal area. [51]
Diagnosis
History
A proper history often helps to rule out other causes of the hair loss like telogen effluvium. The typical history is of chronic hair loss with thinning mainly over the frontal, parietal or vertex areas. The patient might also complain of itching and trichodynia. History of systemic diseases, new medications especially within the previous year should be taken. Family history is usually positive for AGA. Diet is another important aspect of history, to rule out nutrition related effluvium. [48] Lifestyle related enquiries should cover effect of traction, smoking and ultraviolet exposure on AGA, all of which have been implicated as aggravating factors. [52],[53] In female patients, careful attention must be given to assess any associated hormonal dysfunction. [48]
General scalp and hair examination
The scalp is usually normal in AGA, but look for factors which can aggravate AGA like seborrheic dermatitis [54] and photo-damage. [53] The main aim of clinical examination is to identify whether or not the hair loss is patterned.
Pull test
The "pull test" is a simple method to assess the severity of hair loss. [48],[55],[56] About 60 hairs are grasped between the thumb and the index and middle fingers. The hairs are then gently but firmly pulled. A negative test (six or less hairs/less than 10% obtained) indicates normal shedding, whereas a positive test (more than six hairs or 10% obtained) indicates definite active shedding of hair. Shampooing should be withheld for 24 hrs prior to a pull test. In patients with AGA the test is usually negative except in the active phase and that too only in the affected sites like the frontal area. A diffusely positive pull test suggests the possibility of other diagnosis like telogen effluvium.
Trichoscopy
Trichoscopy has emerged as an useful tool in the diagnosis of androgenetic alopecia. Important features of AGA on trichoscopy are hair diameter diversity (HDD) greater than 20% (which corresponds to vellus transformation), perifollicular pigmentation/peripilar sign (the commonest change seen in Asians) and yellow dot [57] [Figure - 1]. The term ′anisotrichosis′ has been proposed to describe the HDD seen in AGA. [58] Rakowska et al., have suggested some criteria which help to differentiate telogen effluvium from AGA in females. Major criteria are ratio of (1) more than four yellow dots in four images (70-fold magnification) in the frontal area in female AGA compare to telogen effluvium (2) lower average hair thickness in the frontal area compared to the occipital area in female AGA (3) more than 10% of thin hairs (below 0.03 mm) in the frontal area in female AGA. Minor criteria include- increased frontal to occipital ratio in AGA of (1) single-hair pilosebaceous units, (2) vellus hairs and (3) perifollicular discoloration. Fulfillment of two major criteria or one major and two minor criteria allows diagnosing AGA in females based on trichoscopy with 98% specificity. [59]
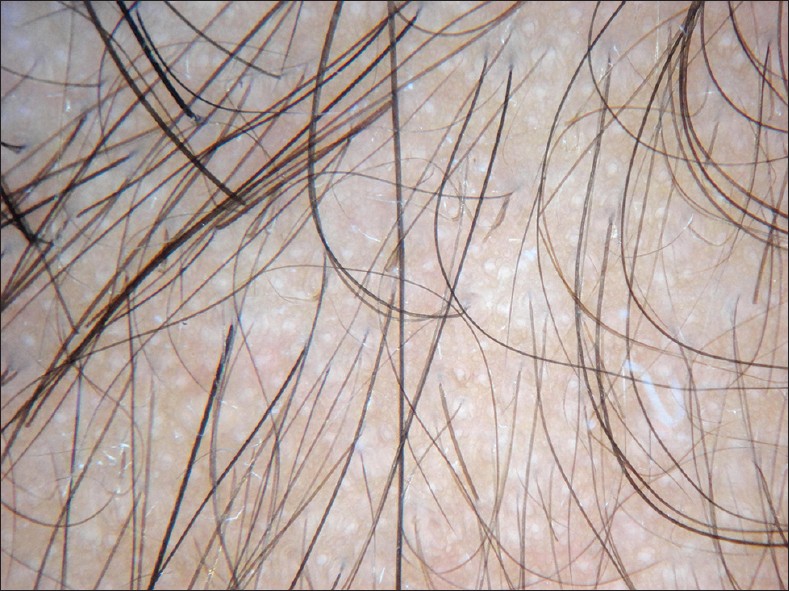 |
| Figure 1: Trichoscopy ×10 showing increase HDD, with prominent vellus transformation, perifollicular pigmentation and yellow spots in AGA |
Trichogram and Photo-trichogram
Trichograms can be used to differentiate between different types of hair loss, but as such have limited application in AGA. The procedure however requires good technical expertise and experience for valid and reliable results. The patient is asked to abstain from washing the hair for five days, following which 60-80 hairs are plucked using a rubber tipped forceps. The hair roots are examined immediately.
Unit - area trichogram - hair follicle density, proportion of anagen fibers, and hair shaft diameter are estimated after plucking hairs in a defined area- usually about 30 mm 2 .
Phototrichogram (PTG) - PTG is a non-invasive method involving production of serial, close-up photographs of specific defined areas to assess hair growth rate, hair follicle density and hair shaft thickness. Variants of this technique include the contrast enhanced PTG and the automated PTG (Trichoscan). One of the essential components of this procedure is to trim the hairs over the selected. [48],[56]
Hair wash test
Rebora et al., have devised a test known as the AGA/TE wash test to distinguish between AGA and TE based on the count of vellus and terminal telogen hairs that are rinsed out on washing the scalp after a five day abstinence from washing and shampooing. The results are given in terms of total telogen hairs and the percentage of telogen vellus hairs. However, this method has definite disadvantages; hair breakage can occur leading to double counting, it is not useful in patients with curly hair and is very time-consuming. [56],[60]
Laboratory Investigations
The general consensus is that extensive laboratory investigations are not required for AGA, especially in males. Some authors recommend testing for Prostate Specific Antigen prior to starting finasteride in men above the age of 45. The main aim of laboratory investigations in women is to rule out any underlying hormonal dysfunction especially polycystic ovarian disease. The tests recommended are - free androgen index test, Dehydroepiandrosterone (DHEAS) and prolactin. Further tests may be considered if necessary to rule out rarer conditions like congenital adrenal hyperplasia. The significance of measuring serum levels of ferritin in AGA is not clear as different studies have produced conflicting results. [48],[61],[62],[63],[64]
Scalp Biopsy
Scalp biopsy is not routinely recommended in AGA because it is an invasive technique. The biopsy is taken from the center of the most affected areas. Biopsies from the bitemporal area are to be avoided as this area tends to have miniaturized hairs even in the absence of AGA. [48],[56] Using a 4 mm punch, two biopsies should be taken ideally - one for transverse sectioning and the other for horizontal sectioning. The horizontal section helps to get an overview of the number, density and morphology of the follicles. The ration of terminal to vellus hairs is normally greater than 7:1, while in AGA it is usually less than 3:1. [65] Other important findings which might be seen in AGA include increased follicular steleae, increased telogen to anagen ratio and a minimal perifollicular lymphohistiocytic infiltrate with or without mild fibrosis around the upper part of the follicle. [66]
Global Photography
A global photograph of a patient with hair loss is a useful tool for follow-up and assessment of treatment response. This requires among other things, a cooperative patient with clean, dry hair and ideally a technician who is able to take the time to comb and prepare the hair precisely the same way at each office visit. The patient should be advised to maintain the same hair style and color. Multiple images should be shot covering all areas of the scalp. The four specific views recommended are the vertex, mid-pattern, frontal, and temporal views. [Figure - 2] The key to good global photography is standardization of image with respect to magnification, position and lighting. Standardization can be best achieved by using a stereotactic imaging apparatus. [48],[67],[68] Global photography is considered to be the most effective method in hair growth evaluation, as the whole scalp hair is evaluated in a standardized way. [69]
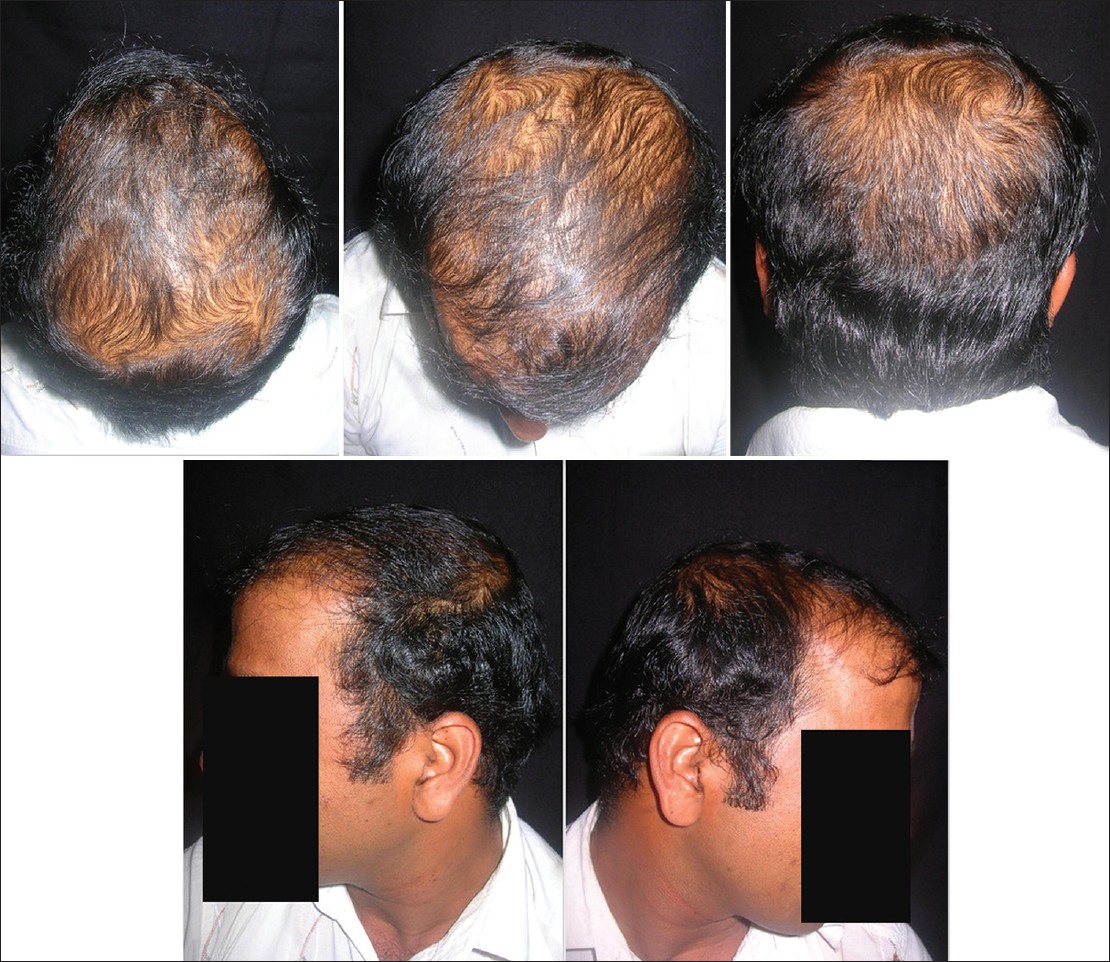 |
| Figure 2: Global photography in AGA (Picture courtesy Dr Ashique KT) |
[Figure - 3] describes the diagnostic algorithm suggest for AGA [48] [Figure - 3].
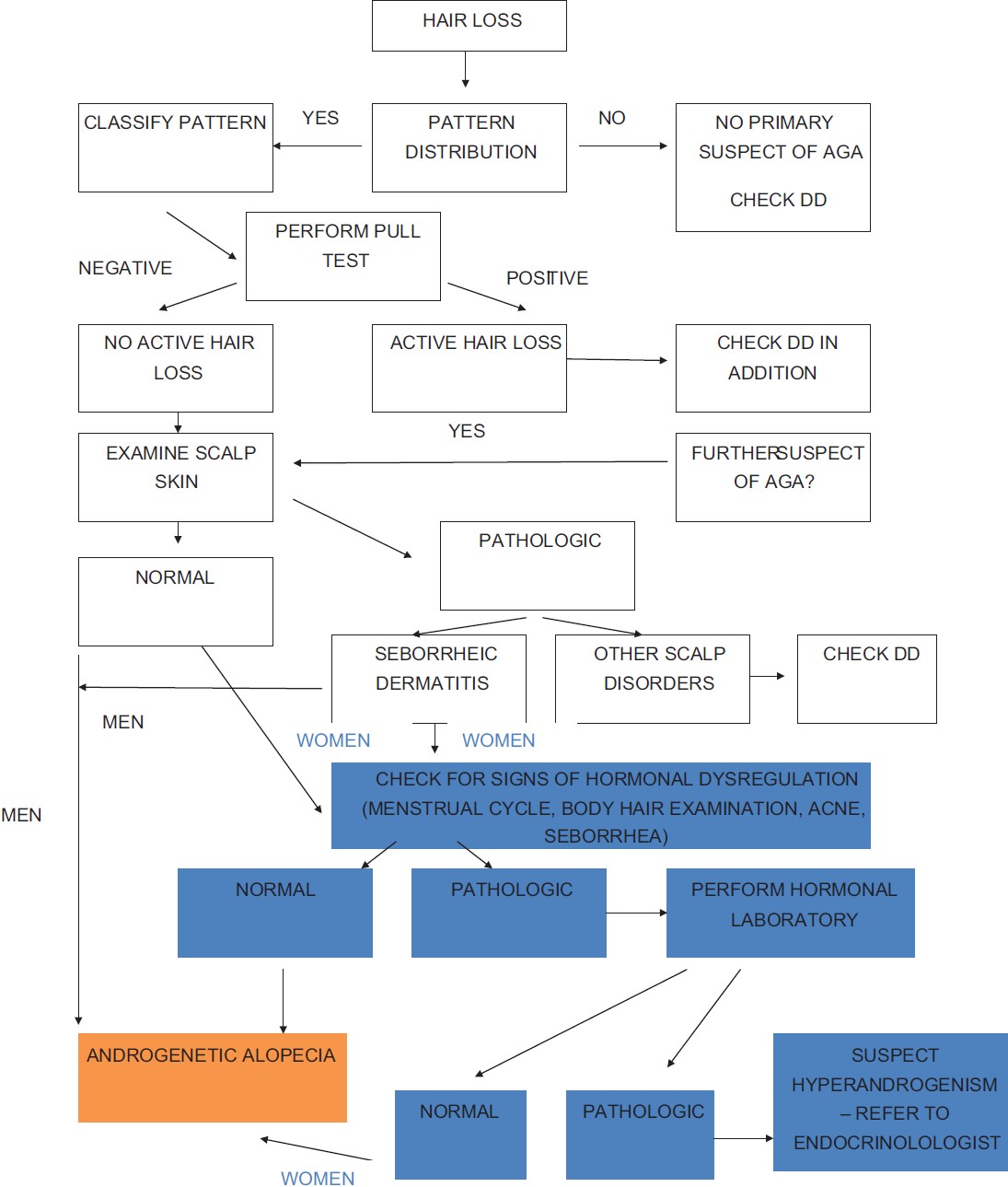 |
| Figure 3: Diagnostic algorithm for androgenetic alopecia (Copyright © 2010, John Wiley and Sons - reused with permission) |
Treatment of AGA
The choice of treatment for AGA depends on various factors including efficacy, practicability, risks and costs. The European Dermatology Forum (EDF) has developed a comprehensive evidence-based S3 guideline for the treatment of AGA which covers all these aspects. [69]
Medical Treatment
Minoxidil
The exact mechanism of action of minoxidil has not been elucidated. Minoxidil is converted to minoxidil sulphate, the active form of the drug which opens ATP-sensitive potassium channels in cell membranes, leading to a vasodilatory effect. While vasodilatation could be one of the possible mechanisms of action, other more important actions of minoxidil on the hair follicles have been suggested including - increased expression of vascular endothelial growth factor (VEGF) mRNA in the dermal papillae, activation of cytoprotective prostaglandin synthase-1, an enzyme that stimulates hair growth and increased expression of hepatocyte growth factor (HGF) m-RNA which is another hair growth promoter. Important recommendations stemming from the meta-analysis by Blumeyer et al., [69] included: Topical 2% and 5% minoxidil solution, 1 ml applied twice daily is effective to prevent progression and improve AGA in males above 18 years. The 5% solution is more effective and the standard formulation (with propylene glycol) is preferred as there is no sufficient evidence for other preparations like the foam preparation or higher concentrations. The response to treatment should be assessed ideally at the end of six months. In female patients there is no sufficient data to recommend the 5% minoxidil solution instead of the 2% solution. Patients should be informed of telogen shedding which is usually seen in the first 8 weeks of therapy. [69] Different studies have shown conflicting results with combination therapy of minoxidil with tretinoin. [70],[71] The most common side effect of topical minoxidil is hypertrichosis. Irritant and allergic contact dermatitis may also occur. Irritation is more common with the 5% solution due to its higher propylene glycol content. [69]
5 alpha reductase inhibitors
The enzyme 5-alpha-reductase converts testosterone to its active form dihydrotestosterone (DHT) and inherited sensitivity of the hair follicles to DHT is one of the etiological factors in AGA. Two types of 5-alpha-reductase are seen in humans. Type I is seen mainly in the liver, skin and scalp while type II predominates in prostate, genitourinary tract and the hair follicle.
Two drugs inhibiting the 5-alpha-reductase used in AGA are finasteride which is a type II 5-alpha-reductase-inhibitor, and dutasteride, which inhibits both type I and type II 5-alpha-reductase.
Important recommendations based on the meta-analysis by Blumeyer et al., [69] include:
Oral finasteride 1 mg a day is recommended to improve or to prevent progression of AGA male patients above 18 years with mild to moderate AGA. The response to treatment should be assessed at 6 months, although in some men it may not become evident until 12 months. Oral dutasteride 0.5 mg a day is another option, but sufficient studies are not available which compare its efficacy to finasteride. There are fewer studies related to the use of finasteride in female patients. Finasteride is contraindicated in pregnancy. Rare adverse effects reported include gynecomastia, reduced libido and erectile dysfunction. Finasteride also reduces PSA level. If treatment is started after the age of 45 years, monitoring of PSA level should be considered. The PSA levels should be double to compensate the reduction due to finasteride, resulting in an interpretation of the test remaining accurate. [69]
Studies have shown that it is not effective in post-menopausal females. It is contraindicated in pregnancy. Topical finasteride is not effective for AGA. [69] Studies in both humans and animals have shown that the combination of minoxidil 2% and finasteride 1 mg is superior to finasteride or minoxidil mono-therapies. [72],[73] Combining hair transplant with finasteride is also considered more effective than hair transplant alone. [74]
Hormonal Treatment
Studies have shown no significant efficacy or role for hormonal therapy - like anti-androgens in male AGA. The only evidence based support for hormonal therapy appears to be the use of cyproterone acetate in female patients with clinical and biochemical evidence of hyperandrogenism. Cyproterone acetate (25-50 mg per day, days 1-10) is usually prescribed together with an oral contraceptive like estradiol. [69],[75] Alfatradiol is a topical estrogen which results in deceleration or stabilization of hair loss. However, studies have shown conflicting results regarding its use in AGA. [76],[77]
Surgery
Hair restoration surgery for AGA essentially involves various forms of hair transplantation. Scalp reduction surgeries are not used frequently at present as a treatment modality for AGA. The efficacy of hair transplantation is based on the principle of donor dominance - androgen insensitive hair follicles keep their properties even when transplanted into scalp areas affected by androgenetic alopecia. Follicles that are not affected by miniaturization are re-distributed over the scalp. Hair transplant is a good option in both males and females with sufficient donor hair. It is recommended to combine hair transplant with oral finasteride for best results. [69],[74]
Hair transplant was first described by Dr. Norman Orentreich [78] in 1959. The back and sides of the scalp are the regions where the hairs remain for the longest period of time. This hair retains its characteristics of growth, color and texture after it is transplanted to the bald area. ′Recipient dominance′ has also been described where the recipient site also has some influence on the transplanted hairs. [79]
′Safe donor area′ is a horse shoe shaped (horizontal) area in the occipital region. The lower limit is the nuchal ridge and the anterior limit is the line drawn vertically from the pre-auricular region. Harvesting is best done at least 2-2.5 cm below the safe superior limit. There must be at least 40FUs/sq cm in the donor area.
Hair is harvested from the donor site using either strip method, follicular unit extraction or a combination of the two.
In the strip method, a strip of scalp is harvested just below the hair follicles. Care must be given to prevent transection of the follicles. The length of the strip is determined by the hair density and the required number of follicular units (FU). After hemostasis of the bed, trichophytic closure can be done where a 1mm ledge is removed from the lower edge of the wound. Wounds are closed with continuous sutures. This allows hairs to grow through the residual scar and scars are therefore more acceptable. [80]
The strips are kept on a slivering board and chilled saline is injected into it to separate out the follicles. The strips are then slivered longitudinally into single FU strips using stereo microscope and then separated into single follicular units. All the while, the grafts are kept moist on wet gauze kept over chilled ringer lactate. Grafts can withstand around 8 h of cold ischemia time when preserved at 4° c.
In Follicular Unit Extraction method, punches are used to extract the FUs. Manual or motorized punches may be used. Blunt punches tend to have lesser transections. This ′stitch less technique′ is preceded by a test harvesting of 10 follicles. If four or more are incomplete, the candidate is termed as FUE negative and a strip method would be more appropriate. [81]
A combined method is where a strip is marked out on the scalp. Then FUE method is used to harvest FUs from above and below the strip. The advantage is that more number of grafts can be obtained for mega-sessions.
Implantation of the follicles is done by making holes on the scalp and implanting FUs into it simultaneously or by making the slits first and then implanting into it later. Implanters like the Choi implanters [82] are available to increase the speed of implantation. Follicular units containing 1-4 hairs are loaded into the implanter. The needle is inserted into the scalp and the plunger pressed to implant the graft. While making the holes, it is important to keep in mind the angle at which the native hairs are coming out of the scalp, as well as the direction of the hairs.
The grafting of follicular units is generally considered the optimum method nowadays, with meticulous stereoscopic microscopic dissection being the "gold standard". [83]
In the immediate postoperative period, the grafts are kept moist with saline spray. The grafted area is dabbed with moist gauze pieces to avoid scab formation. The scalp is washed with shampoo after 48 h. Postoperatively, besides antibiotics and analgesics, minoxidil and finasteride 1mg are given for 6 months. The same medications are advised preoperatively. Minoxidil must be stopped a week prior to the transplant.
The side-effects of hair transplantation surgery are relatively minor consisting of mild pain in the operated areas, swelling which may move down onto the eyes and the formation of scabs over the grafts which take approximately two weeks to resolve. Serious problems of bleeding, scarring, and infection are rare [Figure - 4] and [Figure - 5].
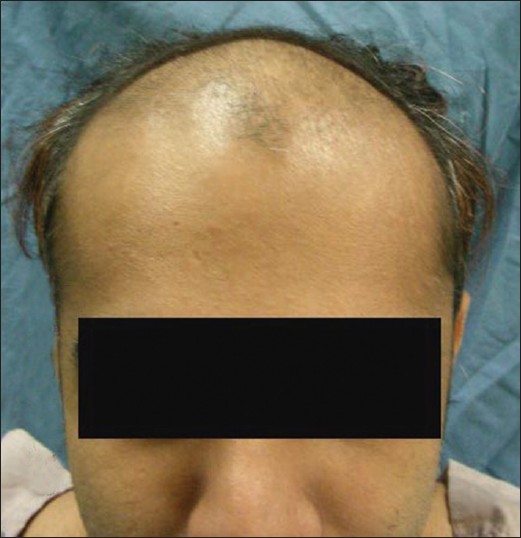 |
| Figure 4: Male pattern hair loss before hair transplant |
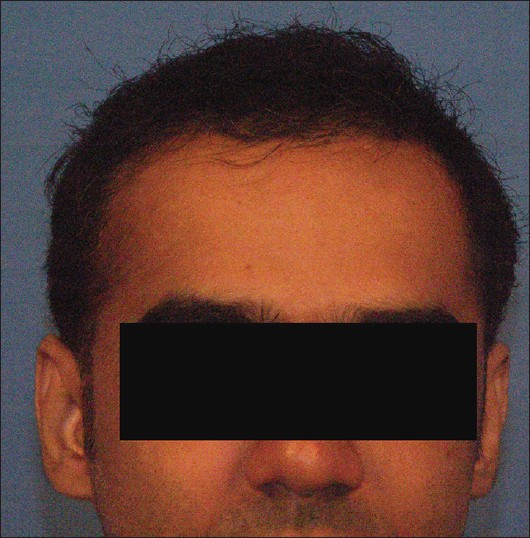 |
| Figure 5: Post hair transplant after one year |
Miscellaneous Treatments
Though not evidence based, a number of other treatment modalities have been tried in AGA. [69] Some of the mechanisms by which these alternative drugs work include: Promotion of hair regrowth by activation of the dermal papillae leading to induction of anagen hair re-growth (Iron supplements, millet seeds, Ginkgo Biloba, Aloe vera, hibiscus, retinoids, cyclosporine), improving the perifollicular vascularization (prostaglandin analogues like latanoprost, aminexil, mesotherapy, benzyl nicotinate, beta-sitosterol), hormonal effects like inhibition of 5-alpha-reductase (polysorbate, green tea, ketoconazole, saw palmetto extract), anti-inflammatory activity (zinc pyrithione, corticosteroids) and improvement of hair follicle nutrition (vitamin supplements, trace elements). [69]
Other topical applications which have been used in AGA, but are not supported by sufficient evidence include - carpronium chloride, t-flavanone, adenosine, Cytopurine/pentadecane and cepharanthine. [84]
Botulinum Toxin
A pilot study on 50 male patients suggested an improvement in AGA with the use of 150 units of botulinum toxin injection in to the muscles around the scalp (the dose divided over 30 injection sites). It has been postulated that botulinum toxin relaxes the scalp muscles thus reducing pressure on the perforating vasculature and improving blood flow and oxygen concentration. This helps improves AGA as low-oxygen environments favor the conversion of testosterone to DHT, while in high oxygen environments it is converted to estradiol. [85]
Lasers and Lights
Paradoxical hair growth after using lasers and lights for hair removal has triggered interest in using these devices as a treatment modality for various types of alopecia, including AGA. The level of evidence for these devices remains poor and there are also some safety concerns. Light of 650-900 nm wavelengths at 5mW has been suggested as an effective option for AGA. [86],[87]
Other Cosmetic Options
Hair extensions, prostheses and wigs are commonly used to improve cosmesis in AGA. Prosthetic hair implantation using synthetic hair is not recommended anymore, mainly because of the high incidence of adverse effects. [84],[88] One of the key factors contributing to the cosmetic appearance of thinning hair is the fiber diameter. A topical combination of caffeine, niacinamide, panthenol, dimethicone and an acrylate polymer (CNDPA) has been shown to improve hair fiber diameter thus helping in enhancing the cosmetic appearance of patients with thinning hair. [89]
Counseling
Considering the psychological impact of AGA, proper patient counseling is an essential aspect of treatment. [1],[4] Important points to keep in mind are to explain the need for life-long treatment in the case of medical treatment and proper selection of patients for surgical intervention. The relatively slow improvement that is expected in the case of medical management should be highlighted. It is important that the patient is given realistic expectations, especially for hair transplantation.
The Future
Research related to the role of stem cells in AGA is likely to open up newer therapeutic options. While stem cell therapy is already being used in many centers, there is no significant data is support clinical use at present. The use of bio-engineered hair follicles derived from stem cells has found to be effective in animal studies and in future this could be a definite option for AGA. [90] Advances in hair transplant procedures, like robotic hair harvesting techniques, could also become more common in the coming days. [91],[92] Research is also focusing on possible newer medical interventions like copper peptides. In vitro studies have demonstrated good results with copper peptides, but at present there is no real scientific evidence to support the same. [93]
Conclusion
Androgenetic alopecia (AGA) is one of the commonest dermatological complaints for which patients seek treatment. AGA can be a source of significant psychological distress to the affected patient. It is important for the dermatologist to understand the process of diagnosis and treatment of AGA. Though effective therapeutic options are limited, AGA continues to remain an area where expanding research is adding more information regarding pathogenesis and newer therapeutic options are being developed accordingly.
| 1. |
Sehgal VN, Kak R, Aggarwal A, Srivastava G, Rajput P. Male pattern androgenetic alopecia in an Indian context: A perspective study. J Eur Acad Dermatol Venereol 2007;21:473-9.
[Google Scholar]
|
| 2. |
Aronson JK, Ramachandran M. The diagnosis of art: Van Gogh and male pattern baldness. J R Soc Med 2009;102:32-3.
[Google Scholar]
|
| 3. |
Stough D, Stenn K, Haber R, Parsley WM, Vogel JE, Whiting DA, et al. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clin Proc 2005;80:1316-22.
[Google Scholar]
|
| 4. |
Kranz D. Young men's coping with androgenetic alopecia: acceptance counts when hair gets thinner. Body Image 2011;8:343-8.
[Google Scholar]
|
| 5. |
Girman CJ, Hartmaier S, Roberts J, Bergfeld W, Waldstreicher J. Patient-perceived importance of negative effects of androgenetic alopecia in women. J Womens Health Gend Based Med 1999;8:1091-5.
[Google Scholar]
|
| 6. |
Wang TL, Zhou C, Shen YW, Wang XY, Ding XL, Tian S, et al. Prevalence of androgenetic alopecia in China: A community-based study in six cities. Br J Dermatol 2010;162:843-7.
[Google Scholar]
|
| 7. |
Shankar DK, Chakravarthi M, Shilpakar R. Male androgenetic alopecia: Population-based study in 1,005 subjects. Int J Trichol 2009;1:131-3.
[Google Scholar]
|
| 8. |
Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol 2001;145:95-9.
[Google Scholar]
|
| 9. |
Grover S. A study of patterns of androgenetic alopecia in men: An Indian perspective. Br J Dermatol 2005;152:572-4.
[Google Scholar]
|
| 10. |
Norwood OT. Incidence of female androgenetic alopecia (female pattern alopecia). Dermatol Surg 2001;27:53-4.
[Google Scholar]
|
| 11. |
Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Invest Dermatol Symp Proc 2005;10:184-9.
[Google Scholar]
|
| 12. |
Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med 1999;341:491-7.
[Google Scholar]
|
| 13. |
Pierard-Franchimont C, Pierard GE. Teloptosis, a turning point in hair shedding biorhythms. Dermatology 2001;203:115-7.
[Google Scholar]
|
| 14. |
Randall VA. The biology of androgenetic alopecia. In: Camacho F, Randall VA, Price VH, editors. Hair and its Disorders: Biology, Pathology and Management. London: Martin Dunitz; 2000. p. 123-36.
[Google Scholar]
|
| 15. |
Roh C, Tao Q, Lyle S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol Genomics 2004;19:207-17.
[Google Scholar]
|
| 16. |
Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004;118:635-48.
[Google Scholar]
|
| 17. |
Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001;104:233-45.
[Google Scholar]
|
| 18. |
Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc 2003;8:46-55.
[Google Scholar]
|
| 19. |
Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 2000;14:1181-5.
[Google Scholar]
|
| 20. |
Kaufman KD. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol Clin 1996;14:697-711.
[Google Scholar]
|
| 21. |
Randall VA. Androgens and human hair growth. Clin Endocrinol 1994;40:439-57.
[Google Scholar]
|
| 22. |
Thornton MJ, Hamada K, Messenger AG, Randall VA. Beard, but not scalp, dermal papilla cells secrete autocrine growth factors in response to testosterone in vitro. J Invest Dermatol 1998;111:727-32.
[Google Scholar]
|
| 23. |
Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g. beard) contain more androgen receptors than those from non-balding areas of the scalp. J Endocrinol 1992;133:141-7.
[Google Scholar]
|
| 24. |
Itami S, Kurata S, Takayasu S. Androgen induction of follicular epithelial cell growth is mediated via insulin like growth factor I from dermal papilla cells. Biochem Biophys Res Commun 1995;212:988-94.
[Google Scholar]
|
| 25. |
Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Identification of androgen-inducible TGF-beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J Investig Dermatol Symp Proc 2003;8:69-71.
[Google Scholar]
|
| 26. |
Philpott MP. The roles of growth factors in hair follicles: Investigations using cultured hair follicles. In: Camacho F, Randall VA, Price VH, editors. Hair and its disorders: Biology, research and management. London: Martin Dunitz; 2001. p. 103-13.
[Google Scholar]
|
| 27. |
Schweikert HU, Wilson JD Regulation of human hair growth by steroid hormones. II. Androstenedione metabolism in isolated hairs. J Clin Endocrinol Metab 1974;39:1012--9.
[Google Scholar]
|
| 28. |
Sawaya ME, Price VH. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol 1997;109:296-300.
[Google Scholar]
|
| 29. |
Sawaya ME, Honig LS, Garland LD, Hsia SL. Delta 5-3 beta-hydroxysteroid dehydrogenase activity in sebaceous glands of scalp in male-pattern baldness. J Invest Dermatol 1988;91:101-5.
[Google Scholar]
|
| 30. |
Paus R. Control of the hair cycle and hair diseases as cycling disorders. Curr Opin Dermatol 1996;3:248-58.
[Google Scholar]
|
| 31. |
Kwack MH, Kim MK, Kim JC, Sung YK. Dickkopf 1 promotes regression of hair follicles. J Invest Dermatol 2012:132:1554-60.
[Google Scholar]
|
| 32. |
Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol 2002;118:216-25.
[Google Scholar]
|
| 33. |
Kitagawa T, Matsuda K, Inui S, Takenaka H, Katoh N, Itami S, et al. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J Clin Endocrinol Metab 2009;94:1288-94.
[Google Scholar]
|
| 34. |
Grisouard J, Mayer D. Specific involvement of glycogen synthase kinase-3 in the function and activity of sex steroid hormone receptors reveals the complexity of their regulation. J Steroid Biochem Mol Biol 2009;117:87-92.
[Google Scholar]
|
| 35. |
Garza LA, Yang CC, Zhao T, Blatt HB, Lee M, He H, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest 2011;121:613-22.
[Google Scholar]
|
| 36. |
Nyholt Dr, Gillepsie NA, Heath AC, Martin NG. Genetic basis of male pattern baldness. J Invest Dermatol 2005;121:1561-4.
[Google Scholar]
|
| 37. |
Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet 2003;33:245-54.
[Google Scholar]
|
| 38. |
Levy-Nissenbaum E, Bar-Natan M, Frydman M, Pras E. Confirmation of the association between male pattern baldness and the androgen receptor gene. Eur J Dermatol 2005;15:339-40.
[Google Scholar]
|
| 39. |
Prodi DA, Pirastu N, Maninchedda G, Sassu A, Picciau A, Palmas MA, et al. EDA2R is associated with androgenetic alopecia. J Invest Dermatol 2008;128:2268-70.
[Google Scholar]
|
| 40. |
Richards JB, Yuan X, Geller F, Waterworth D, Bataille V, Glass D, et al. Male-pattern baldness susceptibility locus at 20p11. Nat Genet 2008;40:1282-4.
[Google Scholar]
|
| 41. |
Brockschmidt FF, Heilmann S, Ellis JA, Eigelshoven S, Hanneken S, Herold C, et al. Susceptibility variants on chromosome 7p21.1 suggest HDAC9 as a new candidate gene for male-pattern baldness. Br J Dermatol 2011;165:1293-302.
[Google Scholar]
|
| 42. |
Hillmer AM, Hanneken S, Ritzmann S, Becker T, Freudenberg J, Brockschmidt FF, et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am J Hum Genet 2005;77:140-8.
[Google Scholar]
|
| 43. |
Ellis JA, Harrap SB. The genetics of androgenetic alopecia. Clin Dermatol 2001;19:149-54.
[Google Scholar]
|
| 44. |
Mahé YF, Michelet JF, Billoni N, Jarrousse F, Buan B, Commo S, et al. Androgenetic alopecia and microinflammation. Int J Dermatol 2000;39:576-84.
[Google Scholar]
|
| 45. |
Philpott MP, Sanders DA, Bowen J, Kealey T. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: A possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol 1996;135:942-8.
[Google Scholar]
|
| 46. |
Norwood OT. Male pattern baldness. Classification and incidence. South Med J 1975;68:1359-65.
[Google Scholar]
|
| 47. |
Hamilton JB. Patterned loss of hair in man: Types and incidence. Ann N Y Acad Sci 1951;53:708-28.
[Google Scholar]
|
| 48. |
Blume-Peytavi U, Blumeyer A, Tosti A, Finner A, Marmol V, Trakatelli M, et al. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol 2011;164:5-15.
[Google Scholar]
|
| 49. |
Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 1977;97:247-54.
[Google Scholar]
|
| 50. |
Sinclair R, Jolley D, Mallari R, Magee J. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol 2004;51:189-99.
[Google Scholar]
|
| 51. |
Lee WS, Oh Y, Ji JH, Park JK, Kim do W, Sim WY, et al. Analysis of familial factors using the basic and specific (BASP) classification in Korean patients with androgenetic alopecia. J Am Acad Dermatol 2011;65:40-7.
[Google Scholar]
|
| 52. |
Su LH, Chen TH. Association of androgenetic alopecia with smoking and its prevalence among Asian men: A community-based survey. Arch Dermatol 2007;143:1401-6.
[Google Scholar]
|
| 53. |
Trüeb RM. Is androgenetic alopecia a photoaggravated dermatosis? Dermatology 2003;207:343-8.
[Google Scholar]
|
| 54. |
Sueki H, Stoudemayer T, Kligman AM, Murphy GF. Quantitative and ultrastructural analysis of inflammatory infiltrates in male pattern alopecia. Acta Derm Venereol 1999;79:347-50.
[Google Scholar]
|
| 55. |
Shapiro J, Wiseman M, Liu H. Practical management of hair loss. Can Fam Physician 2000;46:1469-77.
[Google Scholar]
|
| 56. |
HIllmann K, Blume-Peytavi U. Diagnosis of hair disorders. Semin Cutan Med Surg 2009;28:33-8.
[Google Scholar]
|
| 57. |
Inui S. Trichoscopy for common hair loss diseases: Algorithmic method for diagnosis. J Dermatol 2011;38:71-5.
[Google Scholar]
|
| 58. |
Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol 2012 [In Press].
[Google Scholar]
|
| 59. |
Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: Method standardization and diagnostic criteria. Int J Trichol 2009;1:123-30.
[Google Scholar]
|
| 60. |
Rebora A, Guarrera M, Baldari M, Vecchio F. Distinguishing androgenetic alopecia from chronic telogen effluvium when associated in the same patient: A simple noninvasive method. Arch Dermatol 2005;141:1243-5.
[Google Scholar]
|
| 61. |
Rushton DH, Ramsay ID. The importance of adequate serum ferritin levels during oral cyproterone acetate and ethinyl oestradiol treatment of diffuse androgen-dependent alopecia in women. Clin Endocrinol (Oxf) 1992;36:421-7.
[Google Scholar]
|
| 62. |
Kantor J, Kessler LJ, Brooks DG, Cotsarelis G. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol 2003;121:985-8.
[Google Scholar]
|
| 63. |
Trost LB, Bergfeld WF, Calogeras E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J Am Acad Dermatol 2006;54:824-44.
[Google Scholar]
|
| 64. |
Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology 2008;217:1-6.
[Google Scholar]
|
| 65. |
Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol 1993;28:755-63.
[Google Scholar]
|
| 66. |
Deloche C, de Lacharrière O, Misciali C, Piraccini BM, Vincenzi C, Bastien P, et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res 2004;295:422-8.
[Google Scholar]
|
| 67. |
Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin 1996;14:713-21.
[Google Scholar]
|
| 68. |
Ashique K, Kaliyadan F. Clinical photography for trichology practice: Tips and tricks. Int J Trichol 2011;3:7-13.
[Google Scholar]
|
| 69. |
Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges 2011;9:S1-57.
[Google Scholar]
|
| 70. |
Bazzano GS, Terezakis N, Galen W. Topical tretinoin for hair growth promotion. J Am Acad Dermatol 1986;15:880-3.
[Google Scholar]
|
| 71. |
Shin HS, Won CH, Lee SH, Kwon OS, Kim KH, Eun HC. Efficacy of 5% minoxidil versus combined 5% minoxidil and 0.01% tretinoin for male pattern hair loss: A randomized, double-blind, comparative clinical trial. Am J Clin Dermatol 2007;8:285-90.
[Google Scholar]
|
| 72. |
Khandpur S, Suman M, Reddy BS. Comparative efficacy of various treatment regimens for androgenetic alopecia in men. J Dermatol 2002;29:489-98.
[Google Scholar]
|
| 73. |
Diani AR, Mulholland MJ, Shull KL, Kubicek M F, Johnson GA, Schostarez HJ, et al. Hair growth effects of oral administration of finasteride, a steroid 5 alpha-reductase inhibitor, alone and in combination with topical minoxidil in the balding stumptail macaque. J Clin Endocrinol Metab 1992;74:345-50.
[Google Scholar]
|
| 74. |
Leavitt M, Perez-Meza D, Rao NA, Barusco M, Kaufman KD, Ziering C. Effects of finasteride (1 mg) on hair transplant. Dermatol Surg 2003;31:1268-76.
[Google Scholar]
|
| 75. |
Peereboom-Wynia JD, van der Willigen AH, van Joost T, Stolz E. The effect of cyproterone acetate on hair roots and hair shaft diameter in androgenetic alopecia in females. Acta Derm Venereol 1989;69:395-8.
[Google Scholar]
|
| 76. |
Blume-Peytavi U, Kunte C, Krisp A, Garcia Bartels N, Ellwanger U, Hoffmann R. Comparison of the efficacy and safety of topical minoxidil and topical alfatradiol in the treatment of androgenetic alopecia in women. J Dtsch Dermatol Ges 2007;5:391-5.
[Google Scholar]
|
| 77. |
Orfanos CE, Vogels L. Local therapy of androgenetic alopecia with 17 alpha-estradiol. A controlled, randomized double-blind study. Dermatologica 1980;161:124-32.
[Google Scholar]
|
| 78. |
Orentreich N. Autografts in alopecias and other selected dermatological conditions. Ann NY Acad Sci 1959;83:463-79.
[Google Scholar]
|
| 79. |
Hwang ST, Kim HY, Lee SJ, Lee WJ, Kim do W, Kim JC. Recipient-site influence in hair transplantation: A confirmative study. Dermatol Surg 2009;35:1011-4.
[Google Scholar]
|
| 80. |
Marzola M. Single scar harvesting technique in hair transplantation. In: Haber R, Stough DB, editors. Philadelphia: Elsevier Saunders; 2006. p. 83-5.
[Google Scholar]
|
| 81. |
Rassman W, Harris J, Bernstein R. Follicular Unit Extraction in Hair Transplantation. In: Haber R, Stough DB, editors. Philadelphia: Elsevier Saunders; 2006. p. 133-7.
[Google Scholar]
|
| 82. |
Choi JC, Kim JC. The Choi hair transplanter. In: Stough DB, Haber R, editors. Hair replacement surgical and medical. Philadelphia: Mosby; 1996. p. 125-7.
[Google Scholar]
|
| 83. |
Shiell RC. A review of modern surgical hair restoration techniques. J Cutan Aesthet Surg 2008;1:12-6.
[Google Scholar]
|
| 84. |
Tsuboi R, Itami S, Inui S, Ueki R, Katsuoka K, Kurata S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol 2012;39:113-20.
[Google Scholar]
|
| 85. |
Freund BJ, Schwartz M. Treatment of male pattern baldness with botulinum toxin: A pilot study. Plast Reconstr Surg 2010;126:246e-8.
[Google Scholar]
|
| 86. |
Rangwala S, Rashid RM. Alopecia: A review of laser and light therapies. Dermatol Online J 2012;18:3.
[Google Scholar]
|
| 87. |
Avram MR, Leonard RT Jr, Epstein ES, Williams JL, Bauman AJ. The current role of laser/light sources in the treatment of male and female pattern hair loss. J Cosmet Laser Ther 2007;9:27-8.
[Google Scholar]
|
| 88. |
Lepaw MI. Therapy and histopathology of complications from synthetic fiber implants for hair replacement. A presentation of one hundred cases. J Am Acad Dermatol 1980;3:195-204.
[Google Scholar]
|
| 89. |
Davis MG, Thomas JH, van de Velde S, Boissy Y, Dawson TL Jr, Iveson R, et al. A novel cosmetic approach to treat thinning hair. Br J Dermatol 2011;165:24-30.
[Google Scholar]
|
| 90. |
Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T, et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun 2012;3:784.
[Google Scholar]
|
| 91. |
Lin X, Nakazawa T, Yasuda R, Kobayashi E, Sakuma I, Liao H. Robotic hair harvesting system: A new proposal. Med Image Comput Comput Assist Interv 2011;14:113-20.
[Google Scholar]
|
| 92. |
Rose PT. The latest innovations in hair transplantation. Facial Plast Surg 2011;27:366-77.
[Google Scholar]
|
| 93. |
Pyo HK, Yoo HG, Won CH, Lee SH, Kang YJ, Eun HC, et al. The effect of tripeptide-copper complex on human hair growth in vitro. Arch Pharm Res 2007;30:834-9.
[Google Scholar]
|
Fulltext Views
59,768
PDF downloads
9,419





