Translate this page into:
Anetoderma occurring after hepatitis B vaccination
2 Department of Pathology, Hospital Geral de Santo Antonio, Porto, Portugal,
Correspondence Address:
Marta Teixeira
Department of Dermatology, Trav. F. Sa Carneiro, 44 3� ET, 4460-677, Leca da Palmeira, Portugal
| How to cite this article: Teixeira M, Alves R, Canelhas A, Selores M. Anetoderma occurring after hepatitis B vaccination. Indian J Dermatol Venereol Leprol 2006;72:293-295 |
Abstract
Anetoderma is an elastolytic disorder of unknown origin. To our knowledge, anetoderma secondary to hepatitis B immunization has been described only once in the literature, in two siblings vaccinated at the same time. We describe, what we believe to be an additional case of such a rare disorder in a 21-year-old man. He presented with white spots and papules on his neck, upper limbs, and trunk, that had developed gradually within the last 6 years without any symptoms. The initial lesions were red macules, which gradually enlarged in size and number, becoming pale. Biopsy of a sack-like lesion revealed normal epidermis, and a discrete mononuclear infiltrate throughout the dermis. Association of anetoderma with hepatitis B vaccination is speculated here, as suggested by history of vaccination two weeks prior to the onset of skin eruption, and ruling out other possible causes of anetoderma.

 |
 |
 |
 |
 |
 |
Introduction
Anetoderma is an elastolytic disorder of unknown origin, characterized by circunscribed areas of flaccid skin, which may be depressed macular or papular; the latter can reflect herniation of the subcutaneous tissue.[1] The histology of anetoderma suggests that the basic abnormalilty is focal elastolysis.[2] The mechanism of destruction of the elastic fibers remains unknown. Both cell-mediated and humoral damage have been postulated, but specific anti-elastin reactivity has not yet been shown. Immunologic mechanisms are likely to be important in the pathogenesis of anetoderma, as evidenced by its association with infectious and autoimmune causes. Familial cases have also been described.[3],[4]
Case report
A 21-year-old male was observed for evaluation of white spots and papules on his neck, upper limbs, and trunk, that had developed gradually within the last 6 years without any symptoms. The initial lesions were red macules, which gradually enlarged in size and number, becoming pale. Some of them appeared pouched-out. Our patient consulted us only at this time when he experienced some social constraints, secondary to the skin lesions on exposed areas. He had not noticed any tick bite or migratory erythema. The patient was otherwise in good health, and had no significant past history. No family member was similarly affected.
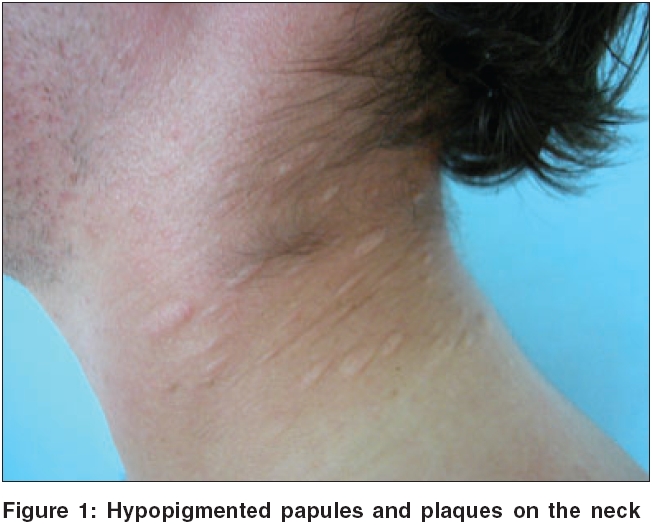
On examination, multiple, grouped, round to elliptical, well-defined, hypopigmented atrophic macules, papules, and plaques with diameter of 5 to 20 mm were found, distributed on the neck, shoulders, upper limbs, scapular, and pectoral regions [Figure - 1][Figure - 2]. Some of the lesions formed soft sack-like projections. The skin surface appeared thinned and wrinkled. The rest of the physical examination, including ocular fundi, was normal. A diagnosis of anetoderma was postulated.
Biopsy of a sack-like lesion revealed normal epidermis, and a discrete mononuclear infiltrate throughout the dermis. Staining with Verhoeff-van Gieson stain showed loss and disruption of dermal elastic fibers in lesional skin, [Figure - 3] but not in a normal- appearing skin fragment. Direct immunofluorescence in both specimens was negative. Polymerase chain reaction studies failed to detect DNA from Borrelia burgdorferi . Further inquiry revealed that the patient finished the third and last dose of hepatitis B virus (HBV) vaccination two weeks before the skin eruption. The vaccine used was Engerix B ® (Smith Kline Beecham), a recombinant vaccine containing HVB surface antigen (HBsAg ). Hepatitis serology revealed negative HBsAg and anti-HBV core antibody, and positive anti-HBV surface antibody, which confirmed immunity to HBV acquired through vaccination. Hepatitis C serology was negative. Urinalysis, full blood count, ESR and biochemical analysis were normal. Antinuclear antibodies and HIV antibodies were not detected. VDRL and TPHA were negative. Immunoglobulin and complement levels were within normal values. Chest radiograph and tuberculin test were normal. At this stage, the diagnosis of anetoderma subsequent to HBV vaccination was contemplated. Our patient refused treatment.
Discussion
Anetoderma has been classified into primary and secondary forms. Primary anetoderma occurs when there is no underlying associated disorder, and it arises on clinically normal skin. True secondary anetoderma implies that the lesion has appeared at the same site as a previous specific skin lesion. Some authors also consider lesions associated with an underlying disease, as a secondary anetoderma; however, in this case, the atrophic areas do not necessarily develop within areas of known inflammation.[1],[2] Some of the reported associations of secondary anetoderma [Table - 1] may be coincidental, but it is probable that many inflammatory diseases may occasionally be complicated by anetoderma.[1],[3],[4]
Immunization with the HBVAg is effective and safe with an estimated incidence of adverse reactions, either local or systemic, of less than 0.1%. These can be divided into local and systemic ones, and may be due to adjuvants or preservatives of the vaccine. Reactions at the injection site, such as pain, erythema, edema, and induration are common and transient, while others, such as nodules or granulomas, are less common, but may persist. Systemic reactions include anaphylactic reactions, fever, headache, arthralgia, myalgia, serum disease, pericarditis, nephrotic syndrome, and neurological and ophthalmologic complications. Cutaneous side effects are rare, and include urticaria, maculopapular rash, erythema nodosum, erythema multiforme, vasculitis, cryoglobulinemia, periarteritis nodosa, systemic or cutaneous lupus erythematosus, and lichen planus or lichenoid reactions.[5]
Anetoderma occurring after HBV vaccination seems to be a very rare event having been described only once in the literature, in two siblings vaccinated at the same time, 2 weeks after the first of three injections of HBV vaccine.[6] In our patient, the development of the lesions, 2 weeks after the third dose of Engerix ®, is consistent with a T-cell mediated immunologic mechanism, as previously postulated by Daoud et al ,[6] although it might reflect a longer sensitization phase. We believe that HBV vaccination might have been the trigger for the onset of this focal elastolysis.
Various treatments for anetoderma have been proposed.[1],[3],[4] However, most have shown variable short-term results, and the long-term outcome was generally unsuccessful.[1],[3],[4] Cosmetically objectionable areas of anetoderma may be excised.We believe that it is important for physicians to report all adverse reactions after HBV vaccination. However the benefits of the vaccine outweigh its very rare hazards.
| 1. |
Maari C, Powell J. Atrophies of connective tisuue. In : Bolognia JL, Jorizzo J, Rapini RP, Horn TD, Mascaro JM, Saurat J, et al , editors. Dermatology. Mosby: St Louis; 2003. p. 1539-48.
[Google Scholar]
|
| 2. |
Bergman R, Friedman-Birnbaum R, Hazaz B, Cohen E, Munichor M, Lichtig C. An immunofluorescence study of primary anetoderma. Clin Exp Dermatol 1990;15:124-30.
[Google Scholar]
|
| 3. |
Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: Part II. Decreased elastic tissue. J Am Acad Dermatol 2004;51:165-85.
[Google Scholar]
|
| 4. |
Venencie PY, Winkelmann RK, Moore BA. Anetoderma. Clinical findings, associations and long-term follow-up evaluations. Arch Dermatol 1984;120:1032-9.
[Google Scholar]
|
| 5. |
McMahon BJ, Helminiak C, Wainwright RB, Bulkow L, Trimble BA, Wainwright K. Frequency of adverse reactions to hepatitis B vaccine in 43,618 persons. Am J Med 1995;98:595-6.
[Google Scholar]
|
| 6. |
Daoud MS, Dicken CH. Anetoderma after hepatitis B immunization in two siblings. J Am Acad Dermatol 1997;36:779-80.
[Google Scholar]
|
Fulltext Views
4,961
PDF downloads
2,057





