Translate this page into:
Angioimmunoblastic T-cell lymphoma
2 Department of Pathology, S. B. K. S. Medical Institute and Research Centre, Sumandeep Vidyapeeth, Piparia, Waghodia, Vadodara, Gujarat, India
Correspondence Address:
Rashmi S Mahajan
Department of Dermatology, Venereology and Leprology, Dhiraj Hospital, Sumandeep Vidyapeeth, Piparia, Waghodia, Vadodara, Gujarat
India
| How to cite this article: Mahajan RS, Vaghani AP, Pasle R K, Bilimoria FE. Angioimmunoblastic T-cell lymphoma. Indian J Dermatol Venereol Leprol 2015;81:315-317 |
Sir,
Angioimmunoblastic T-cell lymphoma is a nodal T-cell lymphoma derived from follicular helper T cells with complex dysregulation of B cells. Extranodal sites, including cutaneous lesions, are well recognized. [1],[2] Cutaneous lesions may manifest as a pruritic maculo-papular eruption mimicking a viral exanthem or drug hypersensitivity reaction, urticaria, erythroderma, erosions, petechiae, purpura, papulo-vesicles, prurigo-like lesions, plaques or as tumor nodules. [3]
A 42-year-old man presented with a history of recurrent abscesses and pruritus for about 8 months accompanied by rapid weight loss, loss of appetite, and generalized weakness. He also had pain and swelling in both wrists for 1 month. There was no history of tuberculosis, diabetes mellitus or exposure to sexually transmitted diseases.
On examination, the patient was pale and poorly nourished. There were multiple abscesses on the scalp, retro-auricular region, trunk and dorsum of hands [Figure - 1]a, b, and d. Deep necrotic ulcers were seen over the buttocks and scrotum [Figure - 1]c. Axillary, inguinal, and femoral lymph nodes were visibly enlarged [Figure - 1]e, about 2 × 3 cm in diameter, soft in consistency, non-tender and mobile. He had generalized xerosis with a few keratotic papules and excoriation marks over the extremities and trunk. Examination of the abdomen revealed non-tender hepatomegaly. There was tenderness and swelling of both knee joints, wrists and hands.
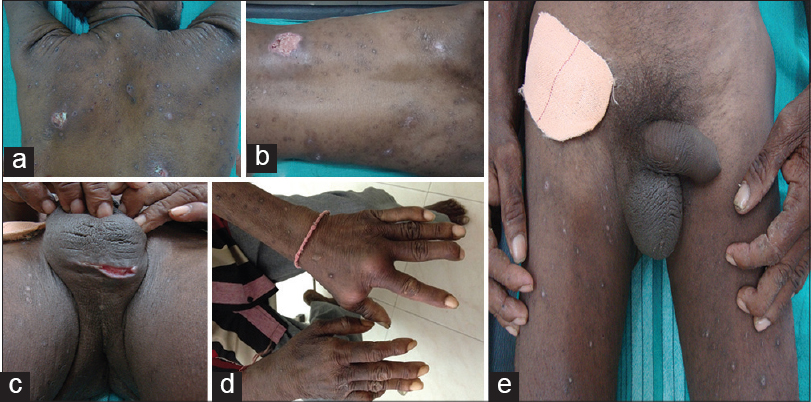 |
| Figure 1: (a and b) Multiple necrotic ulcers over the back, (c) necrotic ulcer over the scrotum, (d) abscesses over the dorsum of left hand, (e) visibly enlarged inguinal and femoral lymph nodes |
Hematological investigations revealed anemia, leukocytosis with eosinophilia and absolute lymphocytosis [hemoglobin (Hb) 10.4 gm%, total count 55,700/cumm], and a raised erythrocyte sedimentation rate (ESR; 150 mm/h). Blood sugar, renal and liver function tests were within normal limits. X-ray of the involved joints was normal. Culture of pus aspirated from the abscesses revealed growth of Staphylococcus aureus.
Skin biopsy revealed an ulcerated epidermis with necrosis and a dense neutrophilic infiltrate. The underlying dermis showed blood vessels infiltrated by neutrophils. Scattered lymphocytes were seen in the upper dermis. Lymph node biopsy revealed diffuse effacement by an interfollicular mixed polymorphous infiltrate of small to medium-sized lymphocytes, plasma cells, eosinophils, epithelioid histiocytes, immunoblasts, and prominent arborizing high endothelial venules [Figure - 2]a-d. Bone marrow biopsy revealed an increase in myeloid, lymphoid, and plasma cells with an increased myeloid: erythroid ratio of 20:1.
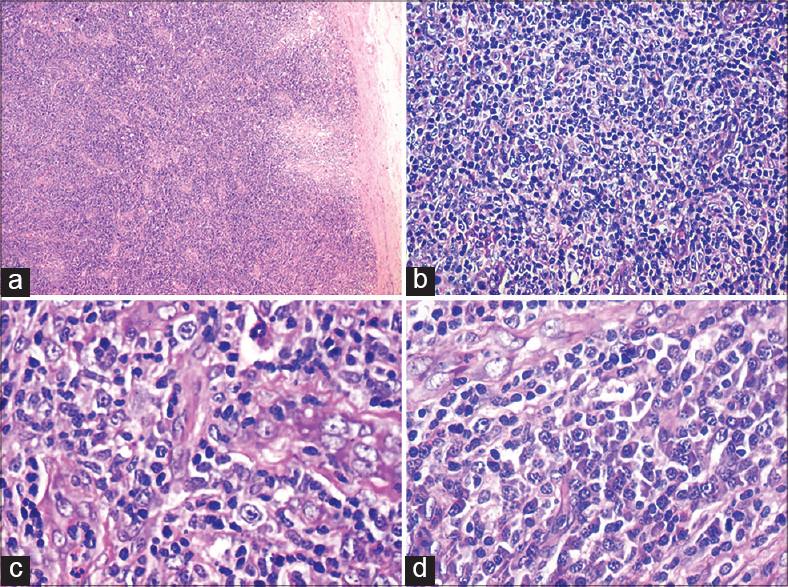 |
| Figure 2: (a) Effacement of normal lymph node architecture, (b) abnormal lymphocyte proliferation with vesicular nuclei and prominent nucleoli, (c) prominent proliferation of high endothelial venules, (d) sheets of plasma cells |
Immunohistochemistry of the lymph node biopsy revealed that the majority of cells expressed CD3 and CD4. Endothelial cells expressed CD31. Follicular dendritic cells expressed CD23, while interfollicular dendritic cells expressed CD1a. Some cells expressed CD20, CD138, and CD23 [Figure - 3]a-e. Immunohistochemistry of the skin biopsy did not reveal atypical cells. Serum electrophoresis showed an increase in alpha-1, alpha-2, beta, and gamma globulins.
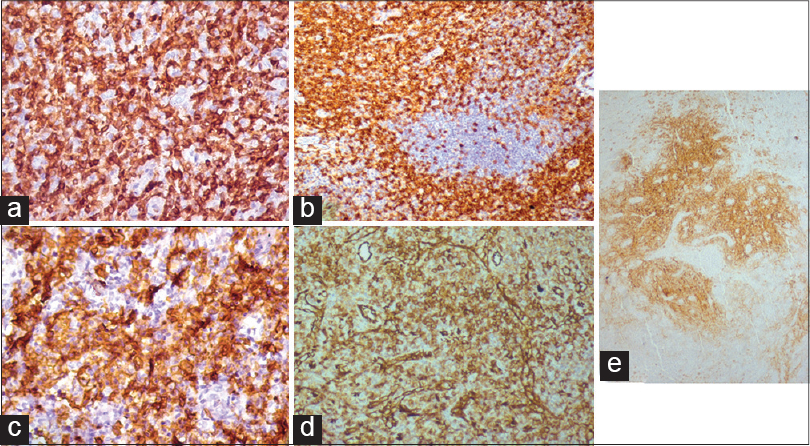 |
| Figure 3: Immunohistochemistry (IHC) showing (a) CD3 expression by T-lymphocytes; (b) CD4 expression by T-lymphocytes; (c) Sheets of plasma cells expressing CD138; (d) High endothelial blood vessels expressing CD31; (e) Follicular dendritic cells decorated by CD23 |
In view of the clinical features and the hematological, histopathological and immunohistochemistry findings, a diagnosis of angioimmunoblastic T-cell lymphoma with dysproteinemia was made.
Angioimmunoblastic T-cell lymphoma is a nodal T-cell lymphoma derived from follicular helper T cells and is associated with dysregulation of B cells. [1] It is a clinico-pathologic syndrome characterized by fever, night sweats, weight loss, generalized lymphadenopathy, hepatomegaly, and splenomegaly. [4] The most common first presentation of the disease is generalized lymphadenopathy. Forty five percent of patients have extranodal cutaneous involvement. [2]
Cutaneous features are frequently observed during the course of this disease. [5],[6] These consist of maculo-papular eruptions, purpura, infiltrated or urticarial plaques, papulo-vesicular lesions, nodules and erythroderma. [3] Facial edema, livedo reticularis, and drug eruption may be seen during chemotherapy. These eruptions regress spontaneously or after corticosteroid treatment. [7] Relapse may also trigger cutaneous features.
The diagnosis can be confirmed by histopathology and immunophenotyping. Lymph node biopsy shows a tumor composed of lymphoid cells with proliferation of arborizing vessels and effacement of nodal architecture. [4],[8] Plasma cells, eosinophils and a dense infiltration of medium to large pleomorphic cells may be seen. Lymphoid cells are positive for CD3, CD4, and CD21 with partial positivity for CD8 and CD30. [1],[9]
The histopathological features are usually non-specific and a small percentage of patients show features suggestive of a cutaneous T-cell lymphoma. [3],[4],[8],[10] Immunophenotypic studies of the dermal lymphoid infiltrates are poorly informative. [9],[10] Martel et al. classified this variant of T-cell lymphoma into four histologic groups as follows: (1) non-specific pattern of mild perivascular infiltrates of eosinophils and lymphocytes with no atypia in the superficial dermis associated with capillary hyperplasia; (2) sparse superficial perivascular infiltrates of atypical lymphocytes with pleomorphic and kidney-shaped nuclei associated with vascular hyperplasia; (3) dense pleomorphic infiltrate composed of atypical lymphocytes in the superficial and deep dermis, suggestive of cutaneous lymphoma; and (4) vasculitis without cellular atypia. [11]
Infectious diseases may be associated with this lymphoma, especially lymphotropic viruses such as Epstein-Barr virus (EBV), which is noted in a majority of lymph nodes. [12]
The molecular alterations underlying the neoplastic transformation of T-helper cells include clonal aberrations in chromosomes 3, 5, and 21. [13],[14]
Treatment with systemic corticosteroids and chemotherapy has been advocated as first line therapy, including cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) based regimens. Monoclonal antibody agents such as alemtuzumab and rituximab added to such regimens may improve the clinical outcomes. [15]
The prognosis of these patients is poor with a mortality rate of 50-75% and a mean survival of 1-2 years from the time of presentation. [3]
Our patient presented with recurrent large abscesses and necrotic ulcers along with severe pruritus, arthritis, fever, significant weight loss, lymphadenopathy and hepato-splenomegaly. He was treated with cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) regimen at a cancer institute.
It is important to recognize that abscess formation and necrotic ulcers may be rare manifestations of angioimmunoblastic T-cell lymphoma in which cutaneous involvement is a poor prognostic sign.
| 1. |
Dunleavy K, Wilson WH, Jaffe ES. Angioimmunoblastic T-cell Lymphoma: Patho-biological insights and clinical implications. Curr Opin Hematol 2007;14:348-53.
[Google Scholar]
|
| 2. |
Lachenal F, Berger F, Ghesquières H, Biron P, Hot A, Callet-Bauchu E, et al. Angioimmunoblastic T-cell lymphoma: Clinical and laboratory features at diagnosis in 77 patients. Medicine 2007;86:282-92.
[Google Scholar]
|
| 3. |
Pangalis GA, Morgan EM, Nathwani BN, Zelman RJ, Kim H, Rappaport H. Angioimmunoblastic lymphadenopathy: Long-term follow-up study. Cancer 1983;52:318-21.
[Google Scholar]
|
| 4. |
Huang CT, Chuang SS. Angioimmunoblastic T-cell Lymphoma with cutaneous involvement: A case report with subtle histologic changes and clonal T-cell proliferation. Arch Pathol Lab Med 2004;128:e122-4.
[Google Scholar]
|
| 5. |
Bernengo MG, Levi L, Zina G. Skin lesions in angioimmunoblastic lymphadenopathy: Histological and immunological studies. Br J Dermatol 1981;104:131-9.
[Google Scholar]
|
| 6. |
Seehafer JR, Goldberg NC, Dicken CH, Su WP. Cutaneous manifestation of angioimmunoblastic lymphadenopathy. Arch Dermatol 1980;116:41-5.
[Google Scholar]
|
| 7. |
Bernstein JE, Soltani K, Lorincz AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol 1979;1:227-32.
[Google Scholar]
|
| 8. |
Freter CE, Cossman J. Angioimmunoblastic lymphadenopathy with dysproteinemia. Semin Oncol 1993;20:627-35.
[Google Scholar]
|
| 9. |
Feller AC, Griesser H, Schilling CV, Wacker HH, Dallenbach F, Bartels H, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol 1988;133:549-56.
[Google Scholar]
|
| 10. |
Mahendran R, Grant JW, Hoggarth CE, Burrows NP. Angioimmunoblastic T-cell Lymphoma with cutaneous involvement. J Eur Acad Dermatol Venereol 2001;15:589-90.
[Google Scholar]
|
| 11. |
Martel P, Laroche L, Courville P, Larroche C, Wechsler J, Lenormand B, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: A clinical, immunohistological, and molecular analysis. Arch Dermatol 2000;136:881-6.
[Google Scholar]
|
| 12. |
Brown HA, Macon WR, Kurtin PJ, Gibson LE. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol 2001;28:432-8.
[Google Scholar]
|
| 13. |
Nelson M, Horsman DE, Weisenburger DD, Gascoyne RD, Dave BJ, Loberiza FR, et al. Cytogenetic abnormalities and clinical correlationsin peripheral T-cell lymphoma. Br J Haematol 2008;141:461-9.
[Google Scholar]
|
| 14. |
Leich E, Haralambieva E, Zettl A, Chott A, Rüdiger T, Höller S, et al. Tissue microarray-based screening for chromosomal breakpoints affecting the T-cell receptor gene loci in mature T-cell lymphomas. J Pathol 2007;213:99-105.
[Google Scholar]
|
| 15. |
National Comprehensive Cancer Network. Guidelines for Non-Hodgkin′s Lymphomas. Version 3. 2012. Available from: http://www.nccn.org/professionals/physicians_gls/f_guidelines.asp. [Last accessed on 2014 Jan 22].
[Google Scholar]
|
Fulltext Views
3,502
PDF downloads
1,794





