Translate this page into:
Antihypertensives in dermatology Part I - Uses of antihypertensives in dermatology
Correspondence Address:
P. S. S. Ranugha
Department of Dermatology and Venereology, JSS Medical College and Hospital, JSS University, Mysore - 570 004, Karnataka
India
| How to cite this article: Ranugha P, Betkerur JB. Antihypertensives in dermatology Part I - Uses of antihypertensives in dermatology. Indian J Dermatol Venereol Leprol 2018;84:6-15 |
Abstract
Hypertension is a global health problem. Antihypertensives are the mainstay of treatment for hypertension. Some of them were accidentally found to be useful in alopecias and infantile hemangiomas and have now become standard treatment for these conditions as well. Antihypertensives are also being studied for other dermatological indications, where they have shown promising efficacy. This review focuses on the dermatological indications for antihypertensives, discussing the drugs that have been tried, as well as their efficacy, dosage, duration of therapy, and adverse effects.Introduction
Antihypertensives(AHTs) are extensively used in the field of medicine, they are classified into different classes based on their mechanism of action viz. calcium channel blockers, beta-blockers, ACE inhibitors, alpha1 blockers, direct vasodilators, diuretics, aldosterone antagonists, angiotensin receptor antagonists and centrally acting drugs.[1] Of the various antihypertensives available now, some are being used effectively in dermatological conditions. Minoxidil, which was used as an emergency drug for hypertension in 1971, was accidentally found to cause increased hair growth when used for a longer duration.[2] Similarly, Leaute-Labreze reported the serendipitous discovery of the dramatic response of infantile hemangiomas to propranolol, when it was used in children with infantile hemangiomas with various cardiac problems like obstructive hypertrophic cardiomyopathy, increased cardiac output etc.[3] The efficacy of these antihypertensives in hemangiomas and androgenetic alopecia has been subsequently proved without doubt in various randomized controlled trials. While they are being used 'off-label' for certain indications, many more are under study and promising results have been shown in various dermatological diseases.
Uses of Antihypertensives in Dermatology
Antihypertensives are being used extensively for the treatment of various dermatological diseases, which are tabulated in [Table - 1].

Nonscarring alopecia
Nonscarring alopecias are more common than scarring alopecias and include male and female pattern hair loss (androgenetic alopecia), alopecia areata, telogen effluvium, trichotillomania, and other less common conditions.
Minoxidil
Minoxidil, originally used as an oral drug for hypertension, was discovered to cause increased hair growth as a side effect, and has since been extensively studied and used in the management of various types of alopecia.
Mechanism of action
Minoxidil is supposed to prolong the duration of the anagen phase and convert vellus hair to terminal hair. However, there is no convincing evidence for this; although, it may prevent or delay follicular miniaturization.[2]
The active form of minoxidil is minoxidil sulphate. Conversion of minoxidil into minoxidil sulfate is higher in hair follicles than in the surrounding skin. The exact biochemical mechanism is not known, but proposed mechanisms include[2],[4],[5]
- ATP-sensitive potassium channel opening, decreasing calcium entry into hair follicle cells, thereby preventing epidermal growth factor (EGF)-induced inhibition of hair growth
- The increased ATP causes release of adenosine, which stimulates vascular endothelial growth factor (VEGF), a promoter of hair growth
- Activation of cytoprotective prostaglandin synthase-1, an enzyme that may stimulate hair growth
- Increased expression of hepatocyte growth factor (HGF) m-RNA, a hair growth promoter.
The strengths available, adverse effects and contraindications for minoxidil are tabulated [Table - 2].[2],[6],[7],[8]

Uses
Male pattern hair loss/androgenetic alopecia and female pattern androgenetic alopecia
While 2% scalp lotion (1988) and subsequently 5% lotion (1997) and 5% foam (2006) were approved for the treatment of male pattern hair loss, 2% minoxidil solution was the only concentration initially approved by the US Food and Drug Association (FDA) and regulatory equivalents (such as the European Dermatology forum) of other countries for treating female pattern hair loss. A few studies found that once-daily 5% minoxidil foam when compared with 2% solution twice daily was efficacious and safe in female pattern hair loss.[9] Five percent minoxidil foam has been recently approved by FDA (2014) and in Canada for use in female pattern hair loss.[10] It is yet to be approved by the Drug Controller General of India (DCGI) for female pattern hair loss.
Minoxidil usually preserves if not reduces the horizontal diameter of alopecia in the crown area. Vellus hair constitutes much of the regrowth in the first 4 months, thereafter terminal hair growth becomes noticeable. It may cause temporary hair shedding during the first month that lasts for 4–6 weeks by inducing anagen from the resting phase. This shedding may be viewed as a clinical indication that the treatment is working. The response to treatment should be assessed at 6 months. If successful, the treatment needs to be continued indefinitely to maintain efficacy.[4] It is important to stress that treatment is long-term and stopping minoxidil will shed all minoxidil-dependent hair within 4–6 months.[11],[12]
Minoxidil is best recommended for mild-to-moderate androgenic alopecia in males (Hamilton–Norwood Grade II–IV).[11],[12] Two studies comparing minoxidil 2% solution applied twice daily and minoxidil 5% solution twice daily in male pattern hair loss showed that the outcome in the minoxidil 5% group was superior to that with minoxidil 2%.[11],[13] Further studies are required to compare the efficacy of minoxidil (5%) solution and foam formulation in male pattern hair loss.
Although topical minoxidil has a good safety profile, efficacy in the overall population remains relatively low at 30–40%. A study by Goren et al. of a sulfotransferase enzyme (SULT1A1) activity assay demonstrated 95% sensitivity and 73% specificity in predicting response to minoxidil treatment in androgenic alopecia.[14]
Minoxidil has also been combined with zinc pyrithione,[15] tretinoin[16] and topical finasteride[2] with variable results.
Alopecia areata
Minoxidil 5% is mainly used as an adjuvant to conventional therapy in alopecia areata. The response is variable depending on the strength of minoxidil used (38% and 81% terminal hair regrowth with 1% and 5% topical minoxidil, respectively).[17] Better results were obtained in a study when it was used in combination with topicalor intralesional steroids, or anthralin.[18] Topical minoxidil is far less effective in alopecia totalis and universalis.[19] Hair loss generally recurs after treatment is stopped because minoxidil does not change perifollicular lymphoid infiltration even in improved cases of alopecia areata.[20]
Congenital hypotrichosis
Minoxidil alone and in combination with tretinoin has been found to be effective in a few cases of congenital alopecia associated with hypohidrotic ectodermal dysplasia.[21],[22] There was an increase in hair density on the scalp without any side effects. In ectodermal dysplasia, there may be a decrease in the maturation of hair follicles rather than a complete absence, which might explain the improvement seen with minoxidil. Bang et al. reported the successful use of minoxidil in a 1-year-old child with temporal triangular alopecia with marked improvement; however there was a recurrence of hair loss after its discontinuation.[23]
Pre- and post-hair transplantation
The use of topical minoxidil in hair transplant patients with viable but suboptimally functioning follicles in the region to be transplanted can add to the density and complement surgical results by slowing down or stopping further hair loss. Results from preliminary uncontrolled clinical trials suggest that topical concentration 2% may speed up regrowth in transplanted follicles, prolong the anagen phase, slow the progression of future hair loss, and reduce post-surgical telogen shedding.[24],[25] Controlled clinical trials are needed to substantiate these preliminary data.
Chemotherapy-induced alopecia
Chemotherapy-induced alopecia is a cause of distress among patients undergoing cancer treatment. Topical 2% minoxidil did show benefit in accelerating hair regrowth in patients who had already finished their chemotherapy regimens, though it did not prevent chemotherapy-induced alopecia when it was used during chemotherapy.[26],[27]
Adverse reactions
Allergic and irritant contact dermatitis are seen in less than 10% of cases. Skin irritation can occur due to minoxidil (particularly with 5%) and propylene glycol but allergic reactions to either of these are rare. Contact dermatitis should be excluded by patch testing. If it is caused by propylene glycol, an alternative vehicle can be used, whereas if irritation and contact dermatitis are due to minoxidil itself, drug withdrawal is unavoidable.[2],[4] Minoxidil is poorly absorbed after topical application. Only 0.3–4.5% reaches systemic circulation and the drug is eliminated within 4 days. Hence, the occurrence of cardiovascular events or headache is very rare.[28]
Facial hypertrichosis and hypertrichosis of the hands was observed in 4% of females using minoxidil in placebo-controlled clinical trials.[29]
Women who already have mild hirsutism are more likely to develop this adverse effect. A few studies have shown this effect with twice- daily applications of 5% solution in females. Hypertrichosis is entirely reversible on discontinuation of the drug.[4],[29]
Central serous chorioretinopathy[30]and erosive pustular dermatosis of scalp[31] have been rarely reported with minoxidil.
Infantile Hemangiomas
Hemangiomas are benign tumors, commonly encountered in infancy and early childhood. While most of them regress spontaneously, a minority of infantile hemangiomas can be problematic or even life-threatening because of their size and/or location. Among the treatments used before the propranolol era, steroids were considered first-line by many authors.
Propranolol
In 2008, Leaute-Labreze et al. published a case series describing the serendipitous discovery that oral propranolol was effective in the treatment of infantile hemangiomas.[3] Within a very short period after its discovery and long before the publication of randomized controlled trials, propranolol became the preferred agent for treatment of complicated infantile hemangiomas. A meta-analysis comparing corticosteroids and propranolol for the treatment of cutaneous infantile hemangiomas demonstrated the corticosteroid studies to have a pooled response rate of 69% versus a rate of 97% with propranolol.[32]
Indications
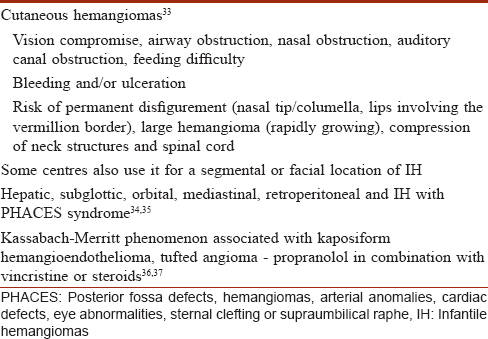
The major indications for propranolol treatment in infantile hemangiomas are described in [Table - 3].[33],[34],[35],[36],[37]
Mechanism of action
Different mechanisms of action have been proposed for the efficacy of propranolol in infantile hemangiomas viz
- Vasoconstriction and lowering of renin[38]
- Inhibition of angiogenesis and proliferation by decreasing expression of VEGF and bFGF (fibroblast growth factor) genes through the downregulation of the rapidly accelerated fibrosarcoma (RAF)–mitogen activated protein kinase pathway in a dose-dependent manner[39]
- Induction of apoptosis of hemangioma endothelial cells through activation of caspase-9 and caspase-3, up-regulation of the pro-apoptotic genes p53 and Bax and down-regulation of the antiapoptotic gene Bcl-Xl[40]
- Accelerated adipogenesis of hemangioma stem cells.[41]
Dosage and monitoring
There is no uniformly accepted protocol for the administration of oral propranolol in infantile hemangiomas. Prospective randomized studies on the optimal modality and duration of therapy, and long-term outcomes after discontinuing propranolol are lacking. The choice between in-hospital and outpatient treatment should be made on a case-by-case basis. McSwiney et al. demonstrated the safety of giving propranolol on a day-care basis with targeted cardiac screening only if necessary.[42] Drolet et al. recommended inpatient care for infants less than 8 weeks of age and those with cardiovascular/respiratory comorbidity[43] In a recent study, it was concluded that routine electrocardiogram (ECG) prior to the start of propranolol therapy may not be necessary in the treatment of children with infantile hemangiomas.[44]
The most commonly used dosage of propranolol is 2 mg/kg/d, although some evidence suggests that infants may respond well at 1–1.5 mg/kg/d, or require as much as 3 mg/kg/d.[34],[43],[45] The usual initial dose is 0.5 mg/kg, gradually increased to a maximum of 3 mg/kg/day. Gunturi et al.[35] conducted a systematic review on propranolol use for infantile hemangiomas and recommended that propranolol solution (prepared by dissolving 10 mg tablet in 5 ml water) should be given thrice daily, with inpatient monitoring for adverse effects (listed below under adverse reactions) for 6 hours after the first dose. Propranolol, in solution form is FDA approved and commercially available as Hemangeol (4.28 mg/ml) for use in children with infantile hemangiomas in USA and the European Union. Parents need to be educated about the warning signs of hypoglycemia and the importance of maintaining a regular feeding schedule. The mean duration of propranolol therapy in a meta-analysis of 41 studies of more than 1200 infants was 6.4 months.[46] However, the optimal length of treatment is yet to be determined prospectively. There are reports of rebound growth of infantile hemangiomas after stopping propranolol,[47] and it seems prudent to continue therapy until beyond the proliferative phase (9–12 months of age) to reduce this. Bertrand et al. suggested that treatment with propranolol should be continued until stabilization has been maintained for 6 months or more, with the duration of therapy depending on the age at which propranolol was initiated.[34] Topical propranolol 1% ointment has been found to be safe and can be tried in patients in whom oral doses cannot be given.[48]
Adverse reactions
In a randomized controlled trial, bradycardia and hypotension were observed to be the most common adverse effects.[48] Others which have been reported include hypoglycemia, hypotonia, wheezing, stridor, sleep disturbances, agitation, nightmares, daytime drowsiness, digestive symptoms like decreased or increased appetite, gastroesophageal reflux, irritability, poor weight gain and acrocyanosis.,[3],[34] There has been a report of allergic contact dermatitis to topical propranolol in a 5-month-old baby.[49] Langley and Pope suggested that caution be exercised in the use of propranolol in children for infantile hemangiomas, considering the evidence from adult volunteer studies regarding its effects on the central nervous system.[50] However, no evidence of psychomotor developmental delay was found in infants with infantile hemangiomas treated with propranolol.[51]
Response to therapy
Better results are obtained if patients are treated during the the early proliferative phase. Late treatment may yield partial improvement.[33] Twenty-four hours after the initiation of propranolol, a change in the color of the lesion from intense red to purple with palpable softening can be observed. Ultrasound examination may show an objective regression in thickness associated with an increase in the resistive index of vascularization of the hemangioma.[3],[48] Symptoms such as dyspnea or hemodynamic abnormalities usually resolve within 48 hours.[45] In the case of orbital infantile hemangiomas with palpebral occlusion, spontaneous ocular opening may be observed within 7 days. Ulcers heal completely within 2 months.[45] Lesions become nearly flat after 6 months of treatment, with persistence of residual skin telangiectasias in some cases.[3]
Risks for relapse
There is rapid recoloration of the infantile hemangiomas on cessation of propranolol therapy, which may be explained by the release of pharmacological vasoconstriction. This observation is common and does not require readministration of propranolol.[34],[48]
In a study by Ahogo et al., 25% of the infants treated with oral propranolol for infantile hemangiomas relapsed. However in half of them the relapse was minor and did not necessitate further therapeutic intervention.[52] They found that the risk of a major relapse (with a true regrowth phase) was 12% in infants treated early (before 5 months of age) with oral propranolol for 6 months. Children at risk of relapse were those with segmental infantile hemangiomas, a deep component to their lesions, and those with infantile hemangiomas involving the head and neck region. Interestingly, they found that the dosage of oral propranolol did not influence the risk of relapse.
A longer duration of therapy may reduce the chances of relapse. A retrospective cohort study of 30 patients with complicated infantile hemangiomas found that the group treated with propranolol for ≤8 months had a 90% relapse rate, whereas ≥12 months of treatment resulted in a 5% relapse rate.[53]
Combination therapies
Propranolol may be combined with prednisolone in selected cases to achieve a rapid response, following which the steroid can be tapered and stopped. In patients with an incomplete response to propranolol, medical therapy may still limit the extent of surgery necessary and thereby aid in an easy and cosmetically acceptable excision.[35]
Other beta blockers and antihypertensives
Other beta blockers including timolol, acetabutolol, nadolol and atenolol have been successfully used in the treatment of infantile hemangiomas. Prospective clinical trials are required to better define the role of each beta blocker according to patient characteristics and lesion type.
In a randomized controlled trial, topical timolol maleate (0.5%) gel or solution applied 2–3 times daily in infants below 6 months of age was found to be efficacious and safe for the treatment of small superficial lesions that were not on mucosal surfaces and were not ulcerated.[54] It has also been used safely in ulcerated hemangiomas and small deep facial hemangiomas in individual reports.[55] In a systematic review and meta-analysis of topical beta blockers for infantile hemangiomas, Ovadia et al. found that the response rates for topical propranolol and topical timolol were not significantly different. They concluded that topically administered beta blockers are effective treatment for superficial infantile hemangiomas that pose fewer adverse effects and should be considered for primary treatment.[56]
Propranolol was found to have a greater benefit than captopril in a randomized controlled trial.[57] More basic and clinical studies are needed to investigate the potential effectiveness of other cardiovascular drugs such as angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers in the management of infantile hemangiomas.
Raynaud's Phenomenon
Antihypertensives which act as direct vasodilators (nitrates, calcium channel blockers) and those which inhibit vasoconstriction (angiotensin receptor blockers, alpha adrenergic receptor blockers) have been tried in the medical management of Raynaud's phenomenon. Dihydropyridine calcium channel blockers are by far the most commonly studied and prescribed class of agents for Raynaud's phenomenon. Calcium channel blockers promote relaxation of vascular smooth muscle cells via inhibition of voltage-gated channels, leading to peripheral vasodilation. A meta-analysis of calcium channel blockers in patients with primary Raynaud's disease revealed significant reductions in frequency and severity of Raynaud attacks.[58] In another meta-analysis of Raynaud's phenomenon secondary to systemic sclerosis, calcium channel blockers reduced attack frequency by 8.3 attacks over 2 weeks and severity by 35%.[59] However, in a head-to-head comparison of 40 mg nifedipine with intravenous iloprost in patients with secondary Raynaud's phenomenon, there was no effect after 1 year of treatment with nifedipine, implying that the beneficial effects due to calcium channel blockers may be lost with long-term treatment.[60]
In clinical practice, calcium channel blockers are the first choice in primary Raynaud's disease and have been suggested for secondary Raynaud's phenomenon as well. Treatment should start with low dosages with titration according to response. In patients with CREST syndrome, calcium channel blockers can reduce sphincter tone in the lower esophagus and hence should be used with caution.[61] The different calcium channel blockers that have been tried include nifedipine (20–120 mg once daily), felodipine (2.5–20 mg once daily), amlodipine (2.5–20 mg once daily), nicardipine and isradipine. Common side effects encountered with calcium channel blockers are hypotension with reflex tachycardia, headache, flushing, dizziness and peripheral edema.[62]
Among the non-dihydropiridine calcium channel blockers, verapamil was found to be ineffective in the management of Raynaud's phenomenon.[63] Diltiazem in a dose of 120 mg/d was found to be useful in both primary and secondary Raynaud's phenomenon, with a more pronounced effect in primary Raynaud's phenomenon.[64] It can also be effective in the long-term treatment of patients with occupation associated Raynaud's syndrome.[65] Diltiazem was found to be ineffective in secondary Raynaud's phenomenon in another study.[66]
Nitrates have been used in both primary and secondary Raynaud's phenomenon in various formulations: topical with transdermal patches, cream or gel, as well as in oral preparations. They have been found to decrease the frequency and severity of attacks in primary and secondary Raynaud's phenomenon, and they may also improve digital ulcers.[67] The efficacy and duration of possible beneficial effects of nitrates are still not clear in patients with secondary Raynaud's phenomenon. Their usage is highly limited by their frequent side effects, mainly headache (80%) and hypotension, irrespective of the route of administration.[61]
Losartan (50 mg/d) significantly decreased the frequency of Raynaud's phenomenon attacks compared to nifedipine after treatment for 15 weeks. Again, beneficial effects were more pronounced in patients with primary Raynaud's phenomenon.[68] Prazosin inhibits the alpha-1 postsynaptic adrenoreceptor with consequent peripheral vasodilation. It was found to be more effective than placebo in a meta-analysis which included patients with secondary Raynaud's phenomenon due to systemic sclerosis. However, several side effects such as nausea, dizziness, headache, palpitations and hypotension limited the use of this drug.[69]
In a systematic review of the effectiveness of various interventions for secondary Raynaud's phenomenon, Huisstede et al.found clear evidence in favor of calcium channel blockers and iloprost (oral and intravenous). For all other interventions, only limited, conflicting, or no evidence was found; hence their place in the therapy of Raynaud's phenomenon is limited to patients who fail to respond adequately to or are unable to tolerate calcium channel blockers.[70]
Flushing
Flushing has been associated with medications, rosacea, menopause, carcinoid syndrome, pheochromocytoma, polycythemia, and mastocytosis, although it can occur without a known cause. There are no known specific treatments available, though beta blockers have suppressed flushing in some patients, particularly when associated with anxiety.[71]
Both oral clonidine 0.05 mg twice daily and transdermal clonidine (0.1 mg weekly) have been found to be effective in treating postmenopausal flushing[72] and flushing in males post-orchidectomy.[73] In carcinoid-induced flushing, clonidine was found to suppress it when given with cimetidine in a case.[74] Alpha adrenoceptor blockers such as phentolamine and phenoxybenzamine have been helpful in improving flushing, diarrhoea, and wheezing in some cases of carcinoid syndrome.[75]
Flushing and erythema related to rosacea
Clonidine may provide modest improvements in flushing in some cases.[76] Rilmenidine, a central hypotensive drug acting specifically on imidazoline receptors and producing no sedation, when given in a dose of 1 mg/d was found to decrease the number of flushing episodes in rosacea.[77] Non-cardioselective beta blockers such as propranolol 40 mg/d may be useful in some cases[71] whereas nadolol is ineffective.[78] Topical brimonidine tartrate gel 0.5%, a selective alpha-2 adrenergic agonist used for ocular hypertension, has been shown to decrease the erythema of rosacea when applied once daily.[79]
Keloids
It has been shown that calcium channel blockerss inhibit the synthesis/secretion of extracellular matrix molecules including collagen, glycosaminoglycans and fibronectin, and increase collagenase.[80] Verapamil could also prohibit proliferative scars by inhibiting TGF-beta1 expression in fibroblasts, as well as by inducing apoptosis.[81]
Similar to other therapeutic options, verapamil injections may be given alone or in combination with surgical excision or other therapies. Depending on the size of the keloid, 0.5–2 ml verapamil is injected per application at a concentration of 2.5 mg/ml. Lawrence noted a cure rate of 55% when they treated earlobe keloids with surgery followed by intralesional verapamil after a week and pressure earrings applied for a minimum of 6 months after excision.[82]
Two randomized studies have been conducted in the past to compare the effects of intralesional verapamil with triamcinolone in hypertrophic scars and keloids.[83],[84] They found that improvements in vascularity, pliability, height and width of the scars were similar with both agents but triamcinolone had a faster effect. Verapamil offers an advantage over triamcinolone with its extremely low cost and fewer adverse effects.
Chilblains (Perniosis)
The efficacy of nifedipine in the treatment of perniosis has been demonstrated in several studies. At a dose of 20–60 mg daily, nifedipine significantly reduces the time to clearance of existing lesions and prevents the development of new chilblains. It also reduces pain, soreness, and irritation in the lesions.[85] Patra et al. compared low-dose nifedepine (10 mg thrice daily) with diltiazem (60 mg thrice daily) in the treatment of chilblains and found low-dose nifedepine to be effective.[86]
Calcinosis Cutis
As calcinosis cutis is a rare syndrome, there is a lack of controlled trials on various therapeutic options. Reiter et al.[87] in their review suggested that, independent of the clinical diagnosis, small calcified deposits or larger localized lesions can be successfully treated by surgical intervention, whereas disseminated calcinosis often requires systemic treatment. There are reports of successful treatment of calcinosis cutis with diltiazem in adult onset and juvenile dermatomyosistis (dose of 2–4 mg/kg/d).[88],[89] While there was partial improvement in a case of calcinosis cutis with Sjogren's syndrome in one study,[90] another study did not find significant improvement with diltiazem when used in a case of systemic sclerosis. The authors attributed this to the usage of a lower dose of diltiazem.[91]
Acne and Hirsuitism
Spironolactone is an aldosterone antagonist and functions as both an androgen receptor blocker and inhibitor of 5-α reductase in acne. There are no currently FDA/EMEA(European Medicines Evaluation Agency)-approved dermatologic indications for spironolactone, and its off-label uses are, among others, female acne, female pattern hair loss, hidradenitis suppurativa and hirsutism [Table - 4][92] It should not be used in pregnant and lactating women and it is not used in men due to the risk of feminization. The average dose used by dermatologists is 50–100 mg daily.[93] Although it has been used successfully in the management of acne, studies on its efficacy are limited by small sample sizes and poor trial design.[94] A Cochrane review concluded that there is some evidence to show that spironolactone is an effective treatment to decrease the degree of hirsutism.[94]

Antiandrogens have been suggested as a possible management strategy in female patients with hidradenitis suppurativa (HS), but there is limited literature on this. Lee and Fischer used spironolactone effectively in 20 cases and advocate it as a low-cost, first-line treatment forHS[95] Spironolactone should not be used with potassium-sparing diuretics, cyclosporine or tacrolimus, due to the risk of hyperkalemia.
Cyclosporine-Induced Hypertension
As cyclosporine is being used extensively by dermatologists for various indications, we shall encounter these problems more frequently, hence this has been included here.
Several mechanisms for cyclosporine-induced hypertension have been proposed i.e. activation of the sympathetic nervous system, endothelin-mediated systemic vasoconstriction, impaired vasodilatation secondary to reduction in prostaglandin and nitric oxide, altered cytosolic calcium translocation, and activation of the renin-angiotensin system (RAS).[96] In a multicentre randomized study, the occurrence of hypertension appeared to be unrelated to the cyclosporine dose,[97] whereas in a systematic review, the effect was found to be dose-related.[98] Cyclosporine-induced hypertension is usually mild and reversible upon dose reduction or discontinuation. Development of hypertension itself is not a contraindication for continuation of cyclosporine as long as it can be controlled with antihypertensives. Calcium channel blockers, particularly of the dihydropyridine group, are preferred because of their effect on smooth muscle vasodilation. Several calcium antagonists, particularly verapamil, nicardipine, and to a lesser extent diltiazem, interfere with cyclosporin metabolism and lead to drug accumulation. Nifedepine, isradipine, and felodipine do not alter the blood levels of cyclosporine, are potent vasodilators, and can be used effectively.[99],[100] It has to be kept in mind that nifedepine can act synergistically with cyclosporine and cause gum hypertrophy.[99]
Beta-blockers have also been used in cyclosporine-induced hypertension either alone or in combination with dihydropyridine calcium channel blockers in transplant recipients.[99] Angiotensin-converting enzyme inhibitors and potassium-sparing diuretics should be used with caution because of their ability to act synergistically with cyclosporine to cause hyperkalemia.[100]
Chronic Nonhealing Ulcers
Beta-2 adrenoreceptors are the dominant receptors present on the surface of keratinocytes. Beta-2 agonists prevent activation of extracellular signal-related kinases (ERKs) which assist wound healing, thereby reducing keratinocyte migration.[101] Beta-2 antagonists transform keratinocytes into a promigratory phenotype but do not directly affect keratinocyte proliferation.[102] They have been shown to increase wound angiogenesis in rats and delay wound contraction. In a randomized controlled trial of 79 people with burns, systemic propranolol (1–1.98 mg/kg) attenuated the hypermetabolic response to burn injury, resulting in shorter healing time, better healing, shorter hospital stays, and smaller wound surface area that required skin graft.[103] Braun et al. reported complete epithelialization of chronic recalcitrant wounds after weekly instillation of 1 drop timolol solution per 2 cm of wound edge, followed by silicone foam dressings for 8 weeks.[104] Similar results have been observed by Tang et al.[105]
Research has found that verapamil reverses calcium-induced inhibition of chemotaxis and adhesion of cultured keratinocytes.[106] Bhaskar et al. found nifedepine and amlodipine to increase tensile strength of wounds in albino rats and overcome steroid-induced depression of wound healing.[107] Acceleration of wound healing was seen in rats with use of biopolymeric nifedepine powder.[108] Azelnidipine was found to enhance wound healing in diabetic rats by increasing nitric oxide production.[109]
Role in Skin Cancers
Nonmelanoma-skin cancers
The RAS(Renin-angiotensin system) is a major regulator of vascular homeostasis. Egami et al. showed that the host angiotensin II type 1 (AT1) receptor plays an important role in angiogenesis and growth of tumor cells engrafted in mice.[110]
The use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers was found to be associated with an approximately two-fold reduced risk of keratinocyte cancers in renal transplant recipients when compared with nonusers in a study. It was suggested that the use of these drugs, should be considered when possible in renal transplant patients with multiple risk factors.[111]
Xiong et al. sought to determine the risk factors for invasive squamous cell carcinomas on the face or ears in a high-risk population and found that the use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers could protect against new invasive squamous cell carcinomas.[112]
Melanoma
De Giorgi et al. observed that beta blocker use is associated with a reduced risk of melanoma recurrence and death,[113] while Koomen et al. concluded that the use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers does not protect against the development of cutaneous melanoma.[114]
Angiotensin II can stimulate the expression of MMP-2, MMP-13, and VEGF (MMP-Matrix-metalloproteinase, VEGF-Vascular endothelial growth factor_) in B16F10 mouse melanoma cells.[115] Otake et al.[116] evaluated the involvement of angiotensin II- dependent pathways in melanoma growth through the pharmacological blockage of AT1 receptors by the antihypertensive drug losartan. They showed that blockage of AT1 receptor signaling may be a promising antitumor strategy, interfering with angiogenesis by decreasing the expression of angiogenic factor receptors. In contrast, Schmidt et al.[117] in a case control study using population-based databases found that long-term angiotensin receptor blocker use and long-term diuretic use may be associated with the risk of developing melanoma and squamous cell carcinomas, respectively. Further studies are needed to evaluate the roles of these drugs in skin cancers.
Cutaneous Infiltration Analgesia
Recently, a solid microstructured transdermal system (sMTS) wherein lidocaine is coated onto microneedles for rapid, prolonged, and safe local analgesia has been developed. Lidocaine rapidly dissolves off the microneedles and into skin such that in 1 min, lidocaine tissue levels needed to cause analgesia are achieved.[118]
Addition of clonidine or related analogs like guanafacine and apraclonidine to the these polymeric microneedles along with lidocaine or prilocaine decreased the systemic absorption rate of the anesthetics from the site of application without impacting their performance or the rapid onset of anesthesia. It also maintained the lidocaine skin concentration above the estimated therapeutic level (100 ng/mg) for 1 h without causing any skin irritation or color change.[119] Co-administration of clonidine with oxybuprocaine, bupivacaine, or dextrorphan was found to increase the potency and duration of infiltrative cutaneous analgesia in rats after a noxious pinprick.[120] Similarly, propranolol when used alone in rats showed more potent and prolonged cutaneous analgesia compared to lidocaine. Propranolol also might prove useful as an adjuvant to lidocaine in producing cutaneous analgesia.[121]
Topical Steroid-Induced Skin Atrophy
Maubec et al. in a recent study found that topical spironolactone gel, when used along with topical corticosteroid, could limit glucocorticoid-induced epidermal atrophy by blocking mineralocorticoid receptors in cultured human skin explants,[122] a concept that needs to be studied further. Nguyen et al. also observed that delayed wound re-epithelialization caused by potent steroid application in mouse skin or cultured human skin explants was rescued by local mineralocorticoid receptor antagonist application.[123]
Conclusion
Antihypertensives are becoming an important part of the dermatologic drug armamentarium. Topical minoxidil and oral propranolol have become the drugs of choice for androgenic alopecia and infantile hemangiomas respectively. Antihypertensives have been used effectively in Raynaud's phenomenon, chilblains, calcinosis cutis, and keloid management either alone or in combination with other therapies. They have also been shown to be effective in chronic nonhealing ulcers and for cutaneous infiltration analgesia; their role in skin cancers remains controversial.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Piascik MT. The Therapy of Hypertension. PHA 824. University of Kentucky. Available from: http://www.uky.edu/~mtp/hypertension_08.htm. [Last cited on 2017 Apr 20].
[Google Scholar]
|
| 2. |
Banka N, Bunagan MJ, Shapiro J. Pattern hair loss in men: Diagnosis and medical treatment. Dermatol Clin 2013;31:129-40.
[Google Scholar]
|
| 3. |
Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649-51.
[Google Scholar]
|
| 4. |
Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges 2011;9 Suppl 6: S1-57.
[Google Scholar]
|
| 5. |
Li M, Marubayashi A, Nakaya Y, Fukui K, Arase S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: Possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J Invest Dermatol 2001;117:1594-600.
[Google Scholar]
|
| 6. |
Uprit S, Kumar Sahu R, Roy A, Pare A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm J 2013;21:379-85.
[Google Scholar]
|
| 7. |
Gelfuso GM, Gratieri T, Simão PS, de Freitas LA, Lopez RF. Chitosan microparticles for sustaining the topical delivery of minoxidil sulphate. J Microencapsul 2011;28:650-8.
[Google Scholar]
|
| 8. |
Gelfuso GM, Gratieri T, Delgado-Charro MB, Guy RH, Vianna Lopez RF. Iontophoresistargeted, follicular delivery of minoxidil sulfate for the treatment of alopecia. J Pharm Sci 2013;102:1488-94.
[Google Scholar]
|
| 9. |
Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol 2011;65:1126-34.e2.
[Google Scholar]
|
| 10. |
Gupta AK, Foley KA. 5% Minoxidil: Treatment for female pattern hair loss. Skin Therapy Lett 2014;19:5-7.
[Google Scholar]
|
| 11. |
Olsen EA, Dunlap FE, Funicella T, Koperski JA, Swinehart JM, Tschen EH, et al. Arandomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol 2002;47:377-85.
[Google Scholar]
|
| 12. |
Olsen EA, Weiner MS, Amara IA, DeLong ER. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol 1990;22:643-6.
[Google Scholar]
|
| 13. |
Price VH, Menefee E, Strauss PC. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J Am Acad Dermatol 1999;41(5 Pt 1):717-21.
[Google Scholar]
|
| 14. |
Goren A, Castano JA, McCoy J, Bermudez F, Lotti T. Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatol Ther 2014;27:171-3.
[Google Scholar]
|
| 15. |
Berger RS, Fu JL, Smiles KA, Turner CB, Schnell BM, Werchowski KM, et al. The effects of minoxidil, 1% pyrithione zinc and a combination of both on hair density: A randomized controlled trial. Br J Dermatol 2003;149:354-62.
[Google Scholar]
|
| 16. |
Shin HS, Won CH, Lee SH, Kwon OS, Kim KH, Eun HC. Efficacy of 5% minoxidil versus combined 5% minoxidil and 0.01% tretinoin for male pattern hair loss: A randomized, double-blind, comparative clinical trial. Am J Clin Dermatol 2007;8:285-90.
[Google Scholar]
|
| 17. |
Fiedler-Weiss VC. Topical minoxidil solution (1% and 5%) in the treatment of alopecia areata. J Am Acad Dermatol 1987;16(3 Pt 2): 745-8.
[Google Scholar]
|
| 18. |
Fiedler VC, Wendrow A, Szpunar GJ, Metzler C, DeVillez RL. Treatment-resistant alopecia areata. Response to combination therapy with minoxidil plus anthralin. Arch Dermatol 1990;126:756-9.
[Google Scholar]
|
| 19. |
Price VH. Double-blind, placebo-controlled evaluation of topical minoxidil in extensive alopecia areata. J Am Acad Dermatol 1987;16(3 Pt 2):730-6.
[Google Scholar]
|
| 20. |
Khoury EL, Price VH, Abdel-Salam MM, Stern M, Greenspan JS. Topical minoxidil in alopecia areata: No effect on the perifollicular lymphoid infiltration. J Invest Dermatol 1992;99:40-7.
[Google Scholar]
|
| 21. |
Melkote S, Dhurat RS, Palav A, Jerajani HR. Alopecia in congenital hidrotic ectodermal dysplasia responding to treatment with a combination of topical minoxidil and tretinoin. Int J Dermatol 2009;48:184-5.
[Google Scholar]
|
| 22. |
Lee HE, Chang IK, Im M, Seo YJ, Lee JH, Lee Y. Topical minoxidil treatment for congenital alopecia in hypohidrotic ectodermal dysplasia. J Am Acad Dermatol 2013;68:e139-40.
[Google Scholar]
|
| 23. |
Bang CY, Byun JW, Kang MJ, Yang BH, Song HJ, Shin J, et al. Successful treatment of temporal triangular alopecia with topical minoxidil. Ann Dermatol 2013;25:387-8.
[Google Scholar]
|
| 24. |
Avram MR, Cole JP, Gandelman M, Haber R, Knudsen R, Leavitt MT, et al. The potential role of minoxidil in the hair transplantation setting. Dermatol Surg 2002;28:894-900.
[Google Scholar]
|
| 25. |
Singh G. The potential role of minoxidil in the hair transplantation setting. Indian J Dermatol Venereol Leprol 1998;64:23-4.
[Google Scholar]
|
| 26. |
Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther 2011;24:432-42.
[Google Scholar]
|
| 27. |
Duvic M, Lemak NA, Valero V, Hymes SR, Farmer KL, Hortobagyi GN, et al. Arandomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol 1996;35:74-8.
[Google Scholar]
|
| 28. |
Shapiro J. Safety of topical minoxidil solution: A one-year, prospective, observational study. J Cutan Med Surg 2003;7:322-9.
[Google Scholar]
|
| 29. |
Dawber RP1, Rundegren J. Hypertrichosis in females applying minoxidil topical solution and in norma controls. J Eur Acad Dermatol Venereol. 2003 May;17 (3):271-5.
[Google Scholar]
|
| 30. |
Guarneri C, Cannavò SP. Erosive pustular dermatosis of the scalp from topical minoxidil 5% solution. Int J Dermatol 2013;52:507-9.
[Google Scholar]
|
| 31. |
Scarinci F, Mezzana P, Pasquini P, Colletti M, Cacciamani A. Central chorioretinopathy associated with topical use of minoxidil 2% for treatment of baldness. Cutan Ocul Toxicol 2012;31:157-9.
[Google Scholar]
|
| 32. |
Izadpanah A, Izadpanah A, Kanevsky J, Belzile E, Schwarz K. Propranolol versus corticosteroids in the treatment of infantile hemangioma: A systematic review and meta-analysis. Plast Reconstr Surg 2013;131:601-13.
[Google Scholar]
|
| 33. |
Solman L, Murabit A, Gnarra M, Harper JI, Syed SB, Glover M. Propranolol for infantile haemangiomas: Single centre experience of 250 cases and proposed therapeutic protocol. Arch Dis Child 2014;99:1132-6.
[Google Scholar]
|
| 34. |
Bertrand J, Sammour R, McCuaig C, Dubois J, Hatami A, Ondrejchak S, et al. Propranolol in the treatment of problematic infantile hemangioma: Review of 35 consecutive patients from a vascular anomalies clinic. J Cutan Med Surg 2012;16:317-23.
[Google Scholar]
|
| 35. |
Gunturi N, Ramgopal S, Balagopal S, Scott JX. Propranolol therapy for infantile hemangioma. Indian Pediatr 2013;50:307-13.
[Google Scholar]
|
| 36. |
Hermans DJ, van Beynum IM, van der Vijver RJ, Kool LJ, de Blalopecia areatauw I, van der Vleuten CJ. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: A new indication for propranolol treatment. J Pediatr Hematol Oncol 2011;33:e171-3.
[Google Scholar]
|
| 37. |
Arunachalam P, Kumar VR, Swathi D. Kasabach-Merritt syndrome with large cutaneous vascular tumors. J Indian Assoc Pediatr Surg 2012;17:33-6.
[Google Scholar]
|
| 38. |
Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br J Dermatol 2010;163:269-74.
[Google Scholar]
|
| 39. |
D'Angelo G, Lee H, Weiner RI. cAMP-dependent protein kinase inhibits the mitogenic action of vascular endothelial growth factor and fibroblast growth factor in capillary endothelial cells by blocking Raf activation. J Cell Biochem 1997;67:353-66.
[Google Scholar]
|
| 40. |
Ji Y, Li K, Xiao X, Zheng S, Xu T, Chen S. Effects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cells. J Pediatr Surg 2012;47:2216-23.
[Google Scholar]
|
| 41. |
Wong A, Hardy KL, Kitajewski AM, Shawber CJ, Kitajewski JK, Wu JK. Propranolol accelerates adipogenesis in hemangioma stem cells and causes apoptosis of hemangioma endothelial cells. Plast Reconstr Surg 2012;130:1012-21.
[Google Scholar]
|
| 42. |
McSwiney E, Murray D, Murphy M. Propranolol therapy for cutaneous infantile haemangiomas initiated safely as a day-case procedure. Eur J Pediatr 2014;173:63-8.
[Google Scholar]
|
| 43. |
Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics 2013;131:128-40.
[Google Scholar]
|
| 44. |
Yarbrough KB, Tollefson MM, Krol AL, Leitenberger SL, Mann JA, MacArthur CJ. Is routine electrocardiography necessary before initiation of propranolol for treatment of infantile hemangiomas? Pediatr Dermatol 2016;33:615-20.
[Google Scholar]
|
| 45. |
Sans V, Dumas de la Roque E, Berge J et al. Propranolol for severe infantile hemangiomas: A follow-up report. Pediatrics. 2009;124:e423–31.
[Google Scholar]
|
| 46. |
Marqueling AL, Oza V, Frieden IJ, Puttgen KB. Propranolol and infantile hemangiomas four years later: A systematic review. Pediatr Dermatol 2013;30:182-91.
[Google Scholar]
|
| 47. |
Shehata N, Powell J, Dubois J, Hatami A, Rousseau E, Ondrejchak S, et al. Late rebound of infantile hemangioma after cessation of oral propranolol. Pediatr Dermatol 2013;30:587-91.
[Google Scholar]
|
| 48. |
Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics 2011;128:e259-66.
[Google Scholar]
|
| 49. |
Bonifazi E, Milano A, Foti C. Allergic contact dermatitis caused by topical propranolol in a 5-month-old baby. Contact Dermatitis 2014;71:250-1.
[Google Scholar]
|
| 50. |
Langley A, Pope E. Propranolol and central nervous system function: Potential implications for paediatric patients with infantile haemangiomas. Br J Dermatol 2015;172:13-23.
[Google Scholar]
|
| 51. |
Moyakine AV, Hermans DJ, Fuijkschot J, van der Vleuten CJ. Propranolol treatment of infantile hemangiomas does not negatively affect psychomotor development. J Am Acad Dermatol 2015;73:341-2.
[Google Scholar]
|
| 52. |
Ahogo CK, Ezzedine K, Prey S, Colona V, Diallo A, Boralevi F, et al. Factors associated with the relapse of infantile haemangiomas in children treated with oral propranolol. Br J Dermatol 2013;169:1252-6.
[Google Scholar]
|
| 53. |
Giachetti A, Garcia-Monaco R, Sojo M, Scacchi MF, Cernadas C, Guerchicoff Lemcke M, et al. Long-term treatment with oral propranolol reduces relapses of infantile hemangiomas. Pediatr Dermatol 2014;31:14-20.
[Google Scholar]
|
| 54. |
Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics 2013;131:e1739-47.
[Google Scholar]
|
| 55. |
Sorrell J, Chamlin SL. Topical timolol 0.5% gel-forming solution for small deep facial infantile hemangiomas. Pediatr Dermatol 2013;30:592-4.
[Google Scholar]
|
| 56. |
Ovadia SA, Landy DC, Cohen ER, Yang EY, Thaller SR. Local administration of ß-blockers for infantile hemangiomas: A systematic review and meta-analysis. Ann Plast Surg 2015;74:256-62.
[Google Scholar]
|
| 57. |
Zaher H, Rasheed H, El-Komy MM, Hegazy RA, Gawdat HI, Abdel Halim DM, et al. Propranolol versus captopril in the treatment of infantile hemangioma (IH): A randomized controlled trial. J Am Acad Dermatol 2016;74:499-505.
[Google Scholar]
|
| 58. |
Thompson AE, Pope JE. Calcium channel blockers for primary Raynaud's phenomenon: A meta-analysis. Rheumatology (Oxford) 2005;44:145-50.
[Google Scholar]
|
| 59. |
Thompson AE, Shea B, Welch V, Fenlon D, Pope JE. Calcium-channel blockers for Raynaud's phenomenon in systemic sclerosis. Arthritis Rheum 2001;44:1841-7.
[Google Scholar]
|
| 60. |
Scorza R, Caronni M, Mascagni B, Berruti V, Bazzi S, Micallef E, et al. Effects of long-term cyclic iloprost therapy in systemic sclerosis with Raynaud's phenomenon. A randomized, controlled study. Clin Exp Rheumatol 2001;19:503-8.
[Google Scholar]
|
| 61. |
Baumhäkel M, Böhm M. Recent achievements in the management of Raynaud's phenomenon. Vasc Health Risk Manag 2010;6:207-14.
[Google Scholar]
|
| 62. |
Pope JE. The diagnosis and treatment of Raynaud's phenomenon: A practical approach. Drugs 2007;67:517-25.
[Google Scholar]
|
| 63. |
Kinney EL, Nicholas GG, Gallo J, Pontoriero C, Zelis R. The treatment of severe Raynaud's phenomenon with verapamil. J Clin Pharmacol 1982;22:74-6.
[Google Scholar]
|
| 64. |
Kahan A, Amor B, Menkes CJ. A randomised double-blind trial of diltiazem in the treatment of Raynaud's phenomenon. Ann Rheum Dis 1985;44:30-3.
[Google Scholar]
|
| 65. |
Matoba T, Chiba M. Effects of diltiazem on occupational Raynaud's syndrome (vibration disease). Angiology 1985;36:850-6.
[Google Scholar]
|
| 66. |
da Costa J, Gomes JA, Espirito Santo J, Queirós M. Inefficacy of diltiazem in the treatment of Raynaud's phenomenon with associated connective tissue disease: A double blind placebo controlled study. J Rheumatol 1987;14:858-9.
[Google Scholar]
|
| 67. |
Teh LS, Manning J, Moore T, Tully MP, O'Reilly D, Jayson MI. Sustained-release transdermal glyceryl trinitrate patches as a treatment for primary and secondary Raynaud's phenomenon. Br J Rheumatol 1995;34:636-41.
[Google Scholar]
|
| 68. |
Dziadzio M, Denton CP, Smith R, Howell K, Blann A, Bowers E, et al. Losartan therapy for Raynaud's phenomenon and scleroderma: Clinical and biochemical findings in a fifteen-week, randomized, parallel-group, controlled trial. Arthritis Rheum 1999;42:2646-55.
[Google Scholar]
|
| 69. |
Pope J, Fenlon D, Thompson A, Shea B, Furst D, Wells G, et al. Prazosin for Raynaud's phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev 2000;2:CD000956.
[Google Scholar]
|
| 70. |
Huisstede BM, Hoogvliet P, Paulis WD, van Middelkoop M, Hausman M, Coert JH, et al. Effectiveness of interventions for secondary Raynaud's phenomenon: A systematic review. Arch Phys Med Rehabil 2011;92:1166-80.
[Google Scholar]
|
| 71. |
Craige H, Cohen JB. Symptomatic treatment of idiopathic and rosacea-associated cutaneous flushing with propranolol. J Am Acad Dermatol 2005;53:881-4.
[Google Scholar]
|
| 72. |
Hammar M, Berg G. Clonidine in the treatment of menopausal flushing. A review of clinical studies. Acta Obstet Gynecol Scand Suppl 1985;132:29-31.
[Google Scholar]
|
| 73. |
Parra RO, Gregory JG. Treatment of post-orchiectomy hot flashes with transdermal administration of clonidine. J Urol 1990;143:753-4.
[Google Scholar]
|
| 74. |
Grosshans E, Michel C, Arcade B, Cribier B. Rilmenidine in rosacea: A double-blind study versus placebo. Ann Dermatol Venereol 1997;124:687-91.
[Google Scholar]
|
| 75. |
Wilkin JK, Rountree CB. Blockade of carcinoid flush with cimetidine and clonidine. Arch Dermatol 1982;118:109-11.
[Google Scholar]
|
| 76. |
Grahame-Smith DG. The carcinoid syndrome. Am J Cardiol 1968;21:376-87.
[Google Scholar]
|
| 77. |
Wilkin JK. Effect of nadolol on flushing reactions in rosacea. J Am Acad Dermatol 1989;20 (2 Pt 1): 202-5.
[Google Scholar]
|
| 78. |
Hsu CC, Lee JY. Pronounced facial flushing and persistent erythema of rosacea effectively treated by carvedilol, a nonselective ß-adrenergic blocker. J Am Acad Dermatol 2012;67:491-3.
[Google Scholar]
|
| 79. |
Fowler J Jr., Jackson M, Moore A, Jarratt M, Jones T, Meadows K, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: Results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol 2013;12:650-6.
[Google Scholar]
|
| 80. |
Copcu E, Sivrioglu N, Oztan Y. Combination of surgery and intralesional verapamil injection in the treatment of the keloid. J Burn Care Rehabil 2004;25:1-7.
[Google Scholar]
|
| 81. |
Xu SJ, Teng JY, Xie J, Shen MQ, Chen DM. Comparison of the mechanisms of intralesional steroid, interferon or verapamil injection in the treatment of proliferative scars. Zhonghua Zheng Xing Wai Ke Za Zhi 2009;25:37-40.
[Google Scholar]
|
| 82. |
Lawrence WT. Treatment of earlobe keloids with surgery plus adjuvant intralesional verapamil and pressure earrings. Ann Plast Surg 1996;37:167-9.
[Google Scholar]
|
| 83. |
Margaret Shanthi FX, Ernest K, Dhanraj P. Comparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloids. Indian J Dermatol Venereol Leprol 2008;74:343-8.
[Google Scholar]
|
| 84. |
Ahuja RB, Chatterjee P. Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns 2014;40:583-8.
[Google Scholar]
|
| 85. |
Rustin MH, Newton JA, Smith NP, Dowd PM. The treatment of chilblains with nifedipine: The results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol 1989;120:267-75.
[Google Scholar]
|
| 86. |
Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: A clinical trial. Indian J Dermatol Venereol Leprol 2003;69:209-11.
[Google Scholar]
|
| 87. |
Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis: Part II. Treatment options. J Am Acad Dermatol 2011;65:15-22.
[Google Scholar]
|
| 88. |
Abdallah-Lotf M, Grasland A, Vinceneux P, Sigal-Grinberg M. Regression of cutis calcinosis with diltiazem in adult dermatomyositis. Eur J Dermatol 2005;15:102-4.
[Google Scholar]
|
| 89. |
Ichiki Y, Akiyama T, Shimozawa N, Suzuki Y, Kondo N, Kitajima Y. An extremely severe case of cutaneous calcinosis with juvenile dermatomyositis, and successful treatment with diltiazem. Br J Dermatol 2001;144:894-7.
[Google Scholar]
|
| 90. |
Vinen CS, Patel S, Bruckner FE. Regression of calcinosis associated with adult dermatomyositis following diltiazem therapy. Rheumatology (Oxford) 2000;39:333-4.
[Google Scholar]
|
| 91. |
Vayssairat M, Hidouche D, Abdoucheli-Baudot N, Gaitz JP. Clinical significance of subcutaneous calcinosis in patients with systemic sclerosis. Does diltiazem induce its regression? Ann Rheum Dis 1998;57:252-4.
[Google Scholar]
|
| 92. |
Salavastru CM, Fritz K, Tiplica GS. Spironolactone in dermatological treatment. On and off label indications. Hautarzt 2013;64:762-7.
[Google Scholar]
|
| 93. |
Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin 2016;34:147-57.
[Google Scholar]
|
| 94. |
Brown J, Farquhar C, Lee O, Toomath R, Jepson RG. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev 2009;2:CD000194.
[Google Scholar]
|
| 95. |
Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol 2015;56:192-6.
[Google Scholar]
|
| 96. |
Niehof M, Borlak J. HNF4alpha dysfunction as a molecular rational for cyclosporine induced hypertension. PLoS One 2011;6:e16319.
[Google Scholar]
|
| 97. |
Laburte C, Grossman R, Abi-Rached J, Abeywickrama KH, Dubertret L. Efficacy and safety of oral cyclosporin A (CyA;Sandimmun) for long-term treatment of chronic severe plaque psoriasis. Br J Dermatol 1994;130:366-75.
[Google Scholar]
|
| 98. |
Robert N, Wong GW, Wright JM. Effect of cyclosporine on blood pressure. Cochrane Database Syst Rev 2010;1:CD007893.
[Google Scholar]
|
| 99. |
Textor SC, Canzanello VJ, Taler SJ, Wilson DJ, Schwartz LL, Augustine JE, et al. Cyclosporine-induced hypertension after transplantation. Mayo Clin Proc 1994;69:1182-93.
[Google Scholar]
|
| 100. |
Taler SJ, Textor SC, Canzanello VJ, Schwartz L. Cyclosporin-induced hypertension: Incidence, pathogenesis and management. Drug Saf 1999;20:437-49.
[Google Scholar]
|
| 101. |
Pullar CE, Grahn JC, Liu W, Isseroff RR. Beta2-adrenergic receptor activation delays wound healing. FASEB J 2006;20:76-86.
[Google Scholar]
|
| 102. |
Souza BR, Santos JS, Costa AM. Blockade of beta1- and beta2-adrenoceptors delays wound contraction and re-epithelialization in rats. Clin Exp Pharmacol Physiol 2006;33:421-30.
[Google Scholar]
|
| 103. |
Mohammadi AA, Bakhshaeekia A, Alibeigi P, Hasheminasab MJ, Tolide-ei HR, Tavakkolian AR, et al. Efficacy of propranolol in wound healing for hospitalized burn patients. J Burn Care Res 2009;30:1013-7.
[Google Scholar]
|
| 104. |
Braun LR, Lamel SA, Richmond NA, Kirsner RS. Topical timolol for recalcitrant wounds. JAMA Dermatol 2013;149:1400-2.
[Google Scholar]
|
| 105. |
Tang JC, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg 2012;38:135-8.
[Google Scholar]
|
| 106. |
Sank A, Chi M, Shima T, Reich R, Martin GR. Increased calcium levels alter cellular and molecular events in wound healing. Surgery 1989; 106:1141-7.
[Google Scholar]
|
| 107. |
Bhaskar HN, Udupa SL, Udupa AL. Effect of nifedipine and amlodipine on dead space wound healing in rats. Indian J Exp Biol 2005;43:294-6.
[Google Scholar]
|
| 108. |
Samy W, Elgindy N, El-Gowelli HM. Biopolymeric nifedipine powder for acceleration of wound healing. Int J Pharm 2012;422:323-31.
[Google Scholar]
|
| 109. |
Bagheri M, Jahromi BM, Mirkhani H, Solhjou Z, Noorafshan A, Zamani A, et al. Azelnidipine, a new calcium channel blocker, promotes skin wound healing in diabetic rats. J Surg Res 2011;169:e101-7.
[Google Scholar]
|
| 110. |
Egami K, Murohara T, Shimada T, Sasaki K, Shintani S, Sugaya T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest 2003;112:67-75.
[Google Scholar]
|
| 111. |
Moscarelli L, Zanazzi M, Mancini G, Rossi E, Caroti L, Rosso G, et al. Keratinocyte cancer prevention with ACE inhibitors, angiotensin receptor blockers or their combination in renal transplant recipients. Clin Nephrol 2010;73:439-45.
[Google Scholar]
|
| 112. |
Xiong MY, Rizzo AE, Cohen TS, Dyer RK, Korgavkar K, Bingham SF, et al. Predictors of squamous cell carcinoma in high-risk patients in the VATTC trial. J Invest Dermatol 2013;133:1521-32.
[Google Scholar]
|
| 113. |
De Giorgi V, Gandini S, Grazzini M, Benemei S, Marchionni N, Geppetti P. Effect of ß-blockers and other antihypertensive drugs on the risk of melanoma recurrence and death. Mayo Clin Proc 2013;88:1196-203.
[Google Scholar]
|
| 114. |
Koomen ER, Herings RM, Guchelalopecia areatar HJ, Nijsten T. Melanoma incidence and exposure to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cancer Epidemiol 2009;33:391-5.
[Google Scholar]
|
| 115. |
Akhavan MM, Karimi M, Ghodrati M, Falahtpishe H. AT1 receptors activation enhances the expression of MMP-2, MMP-13 and VEGF but not MMP-9 in B16F10 melanoma cells. Pak J Biol Sci 2011;14:821-30.
[Google Scholar]
|
| 116. |
Otake AH, Mattar AL, Freitas HC, Machado CM, Nonogaki S, Fujihara CK, et al. Inhibition of angiotensin II receptor 1 limits tumor-associated angiogenesis and attenuates growth of murine melanoma. Cancer Chemother Pharmacol 2010;66:79-87.
[Google Scholar]
|
| 117. |
Schmidt SA, Schmidt M, Mehnert F, Lemeshow S, Sørensen HT. Use of antihypertensive drugs and risk of skin cancer. J Eur Acad Dermatol Venereol 2015;29:1545-54.
[Google Scholar]
|
| 118. |
Zhang Y, Brown K, Siebenaler K, Determan A, Dohmeier D, Hansen K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res 2012;29:170-7.
[Google Scholar]
|
| 119. |
Zhang Y, Siebenaler K, Brown K, Dohmeier D, Hansen K. Adjuvants to prolong the local anesthetic effects of coated microneedle products. Int J Pharm 2012;439:187-92.
[Google Scholar]
|
| 120. |
Chen YW, Chu CC, Chen YC, Hung CH, Hsueh MI, Wang JJ. Clonidine as adjuvant for oxybuprocaine, bupivacaine or dextrorphan has a significant peripheral action in intensifying and prolonging analgesia in response to local dorsal cutaneous noxious pinprick in rats. Neurosci Lett 2011;496:186-90.
[Google Scholar]
|
| 121. |
Chen YW, Chu CC, Chen YC, Hung CH, Wang JJ. Propranolol elicits cutaneous analgesia against skin nociceptive stimuli in rats. Neurosci Lett 2012;524:129-32.
[Google Scholar]
|
| 122. |
Maubec E, Laouénan C, Deschamps L, Nguyen VT, Scheer-Senyarich I, Wackenheim-Jacobs AC, et al. Topical mineralocorticoid receptor blockade limits glucocorticoid-induced epidermal atrophy in human skin. J Invest Dermatol 2015;135:1781-9.
[Google Scholar]
|
| 123. |
Nguyen VT, Farman N, Maubec E, Nassar D, Desposito D, Waeckel L, et al. Re-epithelialization of pathological cutaneous wounds is improved by local mineralocorticoid receptor antagonism. J Invest Dermatol 2016;136:2080-9.
[Google Scholar]
|
Fulltext Views
12,269
PDF downloads
4,910





