Translate this page into:
Apremilast versus betamethasone oral mini-pulse in the treatment of progressive non-segmental vitiligo: A randomised pilot trial
Corresponding author: Dr. Vishal Gupta, Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India. doctor.vishalgupta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma A, Gupta V, Bhatia S, Upadhyay A, Challa A, Gupta S. Apremilast versus betamethasone oral mini-pulse in the treatment of progressive non-segmental vitiligo: A randomised pilot trial. Indian J Dermatol Venereol Leprol. 2025;91:398-401. doi: 10.25259/IJDVL_799_2024
Dear Editor,
Corticosteroid oral mini-pulse (OMP) has been the mainstay of treatment for progressive vitiligo but is limited by adverse effects.1-4 Recently, apremilast was reported to arrest vitiligo activity in all 13 patients in a case series.5 We conducted this study to compare the safety and efficacy of apremilast versus betamethasone OMP in the treatment of progressive non-segmental vitiligo.
The study was conducted in the Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India from November 2020 to March 2023 (CTRI/2020/04/024814) after ethics approval. Adult patients with progressive non-segmental vitiligo (Vitiligo Disease Activity [VIDA] score +4) involving ≥2% body surface area were included after informed consent. A washout period of 2 weeks was given for topical treatment and 4 weeks for phototherapy or systemic therapy. Patients were block (variable size) randomised to receive either apremilast 30 mg twice daily or betamethasone 2.5 mg twice a week (OMP) for 6 months. No topical treatment was allowed during the study. Patients could withdraw from the study at 3 months if vitiligo progression was not arrested. Subjects were assessed bi-weekly for the first month and monthly thereafter. The treatment outcomes included the proportion of patients experiencing a halt in vitiligo progression, change in the number of new vitiligo patches, VIDA score, Vitiligo Area Severity Index (VASI), and percentage repigmentation (assessed by two blinded evaluators), Vitiligo Impact Score (VIS)-22, levels of lesional tissue cytokines (Th1 [IL-2, IFNγ], Th2 [IL-4, IL-13], Th17 [IL-17, IL-22], T-reg [FoxP3]) mRNA expression (with 10 patients in each group), and treatment safety.
Assuming a success rate of 80% for OMP in progressive vitiligo at 6 months4, with a study power of 80%, an alpha error of 5%, a non-inferiority margin of 5%, and a 20% attrition rate, the required sample size was determined to be 262 patients per arm. However, due to cost and time constraints, we planned to conduct this study as a pilot trial.
Statistical analysis was performed as per protocol using Stata software (version 14.0; College Station, TX: StataCorp LP). Continuous variables were compared using the Mann–Whitney U-test or Student’s t-test, while categorical data were analysed with the Chi-square test or Fisher’s exact test. The paired sample t-test was employed to evaluate intra-group differences in continuous variables. Generalised estimating equations were used for longitudinal repeated measures analysis.
Of the 54 patients who received the allocated treatment, 31 (57.4%) completed the study [Figure 1]. The number of patients withdrawing from the study due to side effects (n = 3) or continued disease activity (n = 4) was statistically significantly more in the apremilast arm (6/26 vs 1/28, p = 0.047). The baseline characteristics of patients assigned to both treatment arms, as well as patients who completed the study and those who did not, are summarised in Table 1.

- Consort diagram of the clinical trial.
| Parameter | Apremilast (n = 26) | Betamethasone oral mini-pulse (n = 28) | p value | Patients who completed the protocol (n = 31) | Patients lost to follow-up (n = 23) | p value |
|---|---|---|---|---|---|---|
| Mean age (years) | 31.88 ± 2.26 | 31.43 ± 2.05 | 0.88 | 31.22 ± 11.65 | 32.22 ± 10.42 | 0.75 |
| Sex | 0.58 | 0.90 | ||||
| Male | 13 (50%) | 11 (39.29%) | 14 (45.16%) | 10 (43.48%) | ||
| Female | 13 (50%) | 17 (60.71%) | 17 (54.84%) | 13 (56.52%) | ||
| Duration of disease (years) | 12.05 ± 9.28 | 11.15 ± 8.28 | 0.81 | 12.15 ± 9.98 | 10.82 ± 6.91 | 0.92 |
| Type of vitiligo | 0.99 | 0.22 | ||||
| Acrofacial | 12 (46.15%) | 12 (42.86%) | 16 (51.61%) | 8 (34.78%) | ||
| Generalised | 14 (53.85%) | 16 (57.14%) | 15 (48.39%) | 15 (65.22%) | ||
| Mean VASI | 13.36 ± 15.25 | 15.57 ± 17.08 | 0.42 | 15.28 ± 17.26 | 14.98 ± 19.78 | 0.95 |
| Mean number of new lesions in the past month | 12.31 ± 7.45 | 11.79 ± 9.85 | 0.46 | 12 ± 9.39 | 12.09 ± 7.88 | 0.82 |
| Rapid progressors* | 23 (88.46%) | 25 (89.29%) | 27 (87.10%) | 21 (91.30%) | ||
| History of koebnerisation | 12 (46.15%) | 10 (35.71%) | 0.44 | 15 (48.39%) | 7 (30.43%) | 0.18 |
| Leucotrichia | 17 (65.38%) | 20 (71.43%) | 0.63 | 23 (74.19%) | 14 (60.87%) | 0.30 |
| Family history of vitiligo | 1 (3.85%) | 8 (40%) | 0.03 | 5 (16.13%) | 4 (17.39%) | 0.90 |
| Mean VIS-22 scores | 23.73 ± 12.78 | 26.14 ± 13.47 | 0.53 | 24.54 ± 12.27 | 27.40 ± 12.70 | 0.38 |
At 6 months, 36.4% (n = 4/11) of patients in the apremilast arm had an arrest of vitiligo activity, compared to 60% (n = 12/20) in the OMP arm (p = 0.208) [Figure 2a]. Patients in the OMP arm were 1.95 (95% CI 1.01–3.79, p = 0.047) times more likely to achieve vitiligo arrest during the study period. The mean time to vitiligo arrest was 1.07 ± 0.67 and 2.6 ± 1.78 months in the apremilast and OMP arms, respectively (p = 0.080).
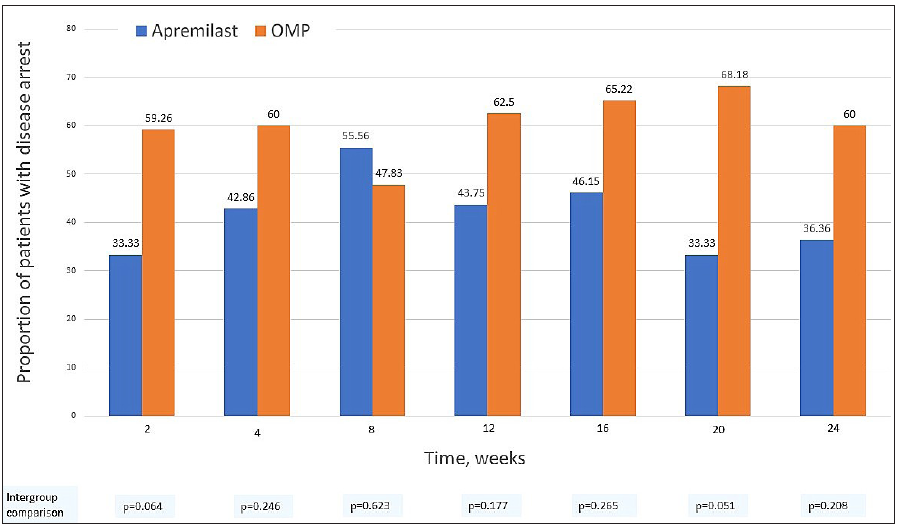
- The proportion of patients achieving vitiligo arrest in apremilast and betamethasone oral mini-pulse treatment arms at each visit.
The mean number of new vitiligo macules decreased statistically significantly at 6 months in both apremilast (12.31 ± 7.45 to 2.18 ± 4.56, p = 0.004) and OMP arms (11.79 ± 9.85 to 1 ± 2.66, p < 0.001) [Figure 2b]. There were 1.25 (95% CI – 3.32–0.83, p = 0.239) lesser new lesions in the OMP arm compared to the apremilast arm during the study period.
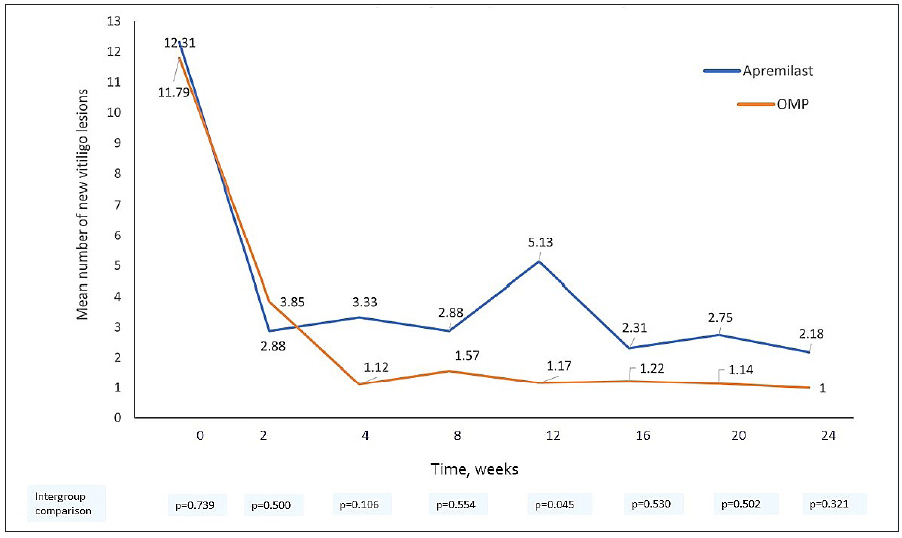
- The mean number of new vitiligo lesions in both apremilast and betamethasone oral mini-pulse treatment arms at each visit.
The proportion of patients with a change in VIDA score at 6 months was also comparable between the two treatment arms (p = 0.337).
There was no statistically significant change in VASI at 6 months from baseline in both the groups (apremilast: 13.36 ± 15.25 vs 13.88 ± 16.37, p = 0.646; OMP: 15.57 ± 17.08 vs 16.38 ± 18.34, p = 0.381). Forty-five per cent of patients (n = 5/11) in the apremilast arm and 65% (n = 13/20) patients in the OMP arm achieved >25% repigmentation (p = 0.685) but none achieved >80% repigmentation.
The mean VIS-22 scores did not change statistically significantly in either arm at 6 months (18 ± 12.86 vs 17.73 ± 10.45 in the apremilast arm, p = 0.894; 26.7 ± 13.74 vs 24.4 ± 11.23 in the OMP arm, p = 0.360).
The mean mRNA expression of Th17 cytokines (IL-17, p = 0.08; IL-22, p = 0.07) in the apremilast arm showed a trend towards statistically significant reduction, while that of IFNγ showed an upward trend (p = 0.06). In the OMP arm, mean IL-17 (p = 0.05) and Foxp3 (p = 0.05) mRNA expression decreased statistically significantly.
Minor adverse events were common in both treatment arms [Table 2]. No patient developed a serious infection in either treatment group. Side effects necessitating discontinuation of therapy were seen in 11.5% (n = 3/26) patients in the apremilast arm (weakness, syncope, nausea, and headache) and none in the OMP arm (p = 0.105).
| Side effects | Apremilast (n = 26) | Betamethasone oral mini-pulse (n = 28) | P value |
|---|---|---|---|
| Overall | 23 (88.46%) | 21 (75%) | 0.298 |
| Gastrointestinal side effects | 16 (61.54%) | 10 (35.71%) | 0.058 |
| Nausea | 12 (46.15%) | 3 (10.71%) | 0.005 |
| Vomiting | 3 (11.54%) | 1 (3.57%) | 0.382 |
| Diarrhoea | 8 (30.77%) | 2 (7.14%) | 0.037 |
| Gastroesophageal reflux | 5 (19.23%) | 8 (28.57%) | 0.422 |
| Headache | 11 (42.31%) | 3 (10.71%) | 0.012 |
| Appetite change | 9 (34.62%) | 8 (28.57%) | 0.023 |
| Increase | Increase: 0 | 6 (21.43%) | |
| Decrease | Decrease: 9 (34.62%) | 2 (7.14%) | |
| Weight change (mean, in kg) | −1 ± 3.86 | +2.33 ± 2.77 | 0.028 |
| Blood pressure >140 systolic and/or >90 diastolic | 5 (19.2%) | 7 (25%) | 0.610 |
| Fasting blood sugar | 0.041 | ||
| >99 mg/dl (pre-diabetic) | 2 (7.69%) | 8 (28.57%) | |
| >125 mg/dl (diabetic) | 0 | 1 (3.57%) | |
| Total leucocyte count> 11,000/µl | 4 (15.38%) | 9 (32.14%) | 0.020 |
| Others | 13 (50%) | 10 (35.7%) | 0.289 |
| Sleep disturbance: 1 (3.85%) | Sleep disturbance:5 (17.86%) | ||
| Malaise/myalgia: 7 (26.92%) | Malaise:1 (3.57%) | ||
| Acute febrile illness: 1 (3.85%) | Acute febrile illness: 2 (7.14%) | ||
| Upper respiratory tract infection: 2 (7.69%) | Scabies: 1 (3.57%) | ||
| Palpitation: 1 (3.85%) | Low mood: 1 (3.57%) | ||
| Altered taste: 1 (3.85%) | Facial puffiness: 2 (7.14%) |
Bold fonts are values that have a statistically significant association.
We found the vitiligo arrest rate to be 60% with OMP at 6 months, consistent with previous reports (44–92%),1-4 while it was 36% in the apremilast arm. This is in contrast to the 100% (n = 13/13) arrest rate in the case series by Majid et al.5 and a randomised trial that reported higher vitiligo arrest rates with add-on apremilast to conventional therapy in 31 patients (94% vs 67%, p = 0.08).6 Despite much lower absolute arrest rates than OMP, apremilast treatment was associated with a quick and sharp decline in the number of new vitiligo macules, but patients had a more fluctuating disease course [Figure 2b]. Treatment with both apremilast and OMP did not produce significant repigmentation which probably explains the lack of improvement in vitiligo-related quality of life. While OMP leads to <75% repigmentation in majority patients,4 repigmentation results with apremilast have been mixed so far.7,8 Low tolerability and high discontinuation rates with apremilast, similar to our results, are reported previously as well.5,6,9 The downward trend in Th17 cytokine signatures in both arms corroborates with the role of IL-17 in vitiligo pathogenesis.10 Kim et al. have previously reported a similar change with apremilast in combination with NB-UVB.11
Study limitations include a small sample size and a high drop-out rate due to the then ongoing COVID-19 pandemic. Nonetheless, our results, generated through robust methodology and recommended outcome measures, can potentially guide further research and are suitable for inclusion in future meta-analysis.
Our results show that apremilast is less effective than OMP in halting vitiligo progression, but it can slow down the disease activity. Given that apremilast is a relatively safe, non-immunosuppressive drug, it may still hold some value in managing vitiligo, which warrants further evaluation.
Ethical approval statement
The research/study was approved by the Institutional Review Board at the All India Institute of Medical Sciences, number Ref IEC-161/06.03.2020, dated 06.03.20.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) Research Grant. Apremilast tablets used in this study were supplied by Glenmark Pharmaceuticals Ltd. The company had no role in study design and conduct of the study, nor collection, management, analysis, and interpretation of the data.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Low-dose oral mini-pulse dexamethasone therapy in progressive unstable vitiligo. J Cutan Med Surg. 2013;17:259-68.
- [CrossRef] [PubMed] [Google Scholar]
- Oral mini-pulse therapy with betamethasone in vitiligo patients having extensive or fast-spreading disease. Int J Dermatol. 1993;32:753-7.
- [CrossRef] [PubMed] [Google Scholar]
- Oral minipulse therapy in vitiligo. Dermatology. 1995;190:251-2.
- [CrossRef] [PubMed] [Google Scholar]
- Oral mini-pulse therapy in vitiligo: a systematic review. Int J Dermatol. 2021;60:868-76.
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast is effective in controlling the progression of adult vitiligo: A case series. Dermatol Ther. 2019;32
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast add-on benefits over conventional drugs (ABCD) in unstable non-segmental vitiligo: A 12-week single-center randomized controlled trial. Cureus. 2023;15
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Apremilast in combination with narrowband UVB in the treatment of vitiligo: A 52-week monocentric prospective randomized placebo-controlled study. J Invest Dermatol. 2020;140:1533-1537.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of apremilast and narrowband ultraviolet B light in the treatment of generalized vitiligo in skin phototypes IV to VI: A randomized split-body pilot study. J Am Acad Dermatol. 2021;85:1657-60.
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast survival and reasons for discontinuation in psoriasis: Five-year experience from a greek tertiary care centre. Dermatol Pract Concept. 2022;12
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The role of IL-17 in vitiligo: A review. Autoimmun Rev. 2016;15:397-404.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Apremilast and narrowband ultraviolet B combination therapy suppresses Th17 axis and promotes melanogenesis in vitiligo skin: a randomized, split-body, pilot study in skin types IV-VI. Arch Dermatol Res. 2023;315:215-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






