Translate this page into:
Assessment of the therapeutic benefit of dexamethasone cyclophosphamide pulse versus only oral cyclophosphamide in phase II of the dexamethasone cyclophosphamide pulse therapy: A preliminary prospective randomized controlled study
2 Department of Immunopathology, Post-Graduate Institute of Medical Education and Research, Chandigarh, India
3 Department of Dermatology, Kurume University School of Medicine, and Kurume University Institute of Cutaneous Cell Biology, Fukuoka, Japan
Correspondence Address:
Amrinder J Kanwar
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh - 160 012
India
| How to cite this article: Parmar NV, Kanwar AJ, Minz RW, Parsad D, Vinay K, Tsuruta D, Ishii N, Hashimoto T. Assessment of the therapeutic benefit of dexamethasone cyclophosphamide pulse versus only oral cyclophosphamide in phase II of the dexamethasone cyclophosphamide pulse therapy: A preliminary prospective randomized controlled study. Indian J Dermatol Venereol Leprol 2013;79:70-76 |
Abstract
Background: Dexamethasone cyclophosphamide pulse (DCP) therapy is an established mode of treatment for pemphigus in India. Aims: To assess the therapeutic benefit of additional DCPs (phase II, consolidation phase) versus immediate oral cyclophosphamide, usually used in phase III (maintenance phase), after initial DCP therapy (phase I) and to assess which laboratory test (DIF or ELISA) will reflect the clinical relapse best. Methods: Nineteen newly recruited patients of pemphigus vulgaris (PV) received monthly DCPs in phase I and were then randomized into two groups. Group A (10 patients) received monthly DCPs for nine months and Group B (nine patients) received only oral cyclophosphamide for nine months. Direct immunofluorescence (DIF) and enzyme-linked immunosorbent assay (ELISA) were tested before starting DCP regimen, and at 0,3,6,9 months after randomization. Results: Clinical relapse by the end of follow-up period occurred in only one patient in each group. In these cases, DIF became (again) positive before the relapse. No statistically significant difference between the two groups was found at three, six and nine months by ELISA indices and DIF grading. Conclusion: Although the DCP regimen is the standard therapy for pemphigus in India, we found no difference in the clinical outcome between patients receiving nine DCPs in phase II and patients shifted directly from phase I to III. Periodic testing using DIF and Dsg ELISA were found to be useful to monitor disease activity and predict a relapse. Further large scale studies are required to assess if patients can be shifted directly from phase I to III and maintained only on oral cyclophosphamide.Introduction
Pemphigus comprises of a group of autoimmune intraepidermal blistering disorders of skin and mucous membranes caused by pathogenic immunoglobulin(Ig)G autoantibodies against transmembrane desmosomal glycoproteins, desmoglein (Dsg) 1 and Dsg 3. Pemphigus is divided into two major types: pemphigus vulgaris (PV) and pemphigus foliaceous (PF). Mucosal dominant type PV targets only Dsg3 and mucocutaneous type PV targets both Dsg1 and Dsg3, while PF targets only Dsg1.
The mainstay of treatment of pemphigus in India is the dexamethasone cyclophosphamide pulse (DCP) regimen described by Pasricha and Ramji in 1984. [1] In this regimen, after patients achieve clinical remission using DCPs (phase I), an additional nine DCPs are given as consolidation phase (phase II), followed by only oral cyclophosphamide for nine months as maintenance (phase III).
The aim of this study was to assess the necessity of DCPs in phase II versus a direct shift from phase I to III and to study the utility of direct immunofluorescence (DIF) and Dsg enzyme-linked immunosorbent assay (ELISA) in predicting clinical relapse. The primary outcome measure was the incidence of relapse and secondary outcome measures were DIF results and indices of Dsg ELISA. Generally, immunofluorescence has been extensively used in the diagnosis of pemphigus. In recent years, Dsg ELISAs have been shown to be highly sensitive and specific quantitative tests in the diagnosis of pemphigus. [2] However, their usefulness in clinical remission has not been extensively studied. To the best of our knowledge this is the first study wherein both DIF and ELISA used simultaneously in pemphigus patients in clinical remission, to judge their benefit for treatment decisions.
Methods
Patients
This was a prospective, single centre, randomized study carried out in a tertiary care setting in north India. Thirty two consecutive patients of PV were screened from February 2009 to August 2009. Diagnosis for new PV patients was made on the basis of classical clinical presentation of oral mucosal erosions and flaccid cutaneous blisters, skin histopathology of intraepidermal acantholysis and DIF demonstrating the deposition of IgG and C3 complement component on epidermal keratinocyte cell surfaces. Patients of either sex, aged between 18 and 60 years and who completed phase I of DCP regimen were recruited for the study and randomized to two different regimens. Twenty-three patients fulfilled the above criteria, but three denied informed consent one patient was lost to follow-up after phase I, thus 19 patients were included in the study and randomized to the two different regimens. The patients were informed about the need of four skin biopsies required for the study and a written informed consent was taken from each patient prior to the start of the study.
Dexamethasone cyclophosphamide pulse regimen
Dexamethasone cyclophosphamide pulse (DCP) regimen is arbitrarily divided into four phases. In phase I, patients receive 100mg of dexamethasone dissolved in 250ml of 5% dextrose for three consecutive days every four weeks. Cyclophosphamide 500mg is administered as a slow intravenous bolus on day 2 of DCP. In the intervening period between two pulses, patients take only oral cyclophosphamide 50 mg daily. When a complete clinical remission is achieved, patients are shifted to phase II. Nine additional pulses are given in phase II every four weeks and cyclophosphamide 50mg is continued in the intervening period. If the patients continue to be in clinical remission at the end of nine pulses they enter phase III where only oral cyclophosphamide 50mg is given for nine months. Subsequently, patients enter phase IV which constitutes post-treatment follow-up. [3] Thus the duration of this regimen varies from patient to patient, as the length of phase I is variable. In this study, clinical remission conformed to complete remission on therapy according to the consensus statement from the International Pemphigus Committee. [4]
Study design [Figure - 1]
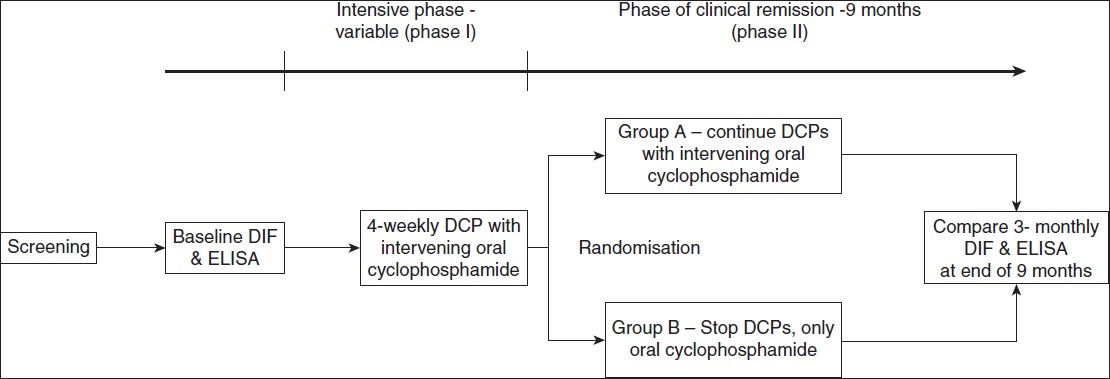 |
| Figure 1: Study design |
After obtaining ethical clearance from the institutional ethical committee, the standard DCP regimen described above was administered to all 20 patients. The diagnostic pre-treatment DIF was taken at the baseline and blood sample for ELISA was collected prior to starting the regimen. All patients then received phase I of the regimen until they achieved clinical remission. Nineteen patients completed phase I of DCP regimen, one patient was lost to follow-up prior to completion of phase I of DCP. These patients were then randomized into two groups using a random number table. Ten patients in group A received the standard phase II DCP regimen (DCPs + cyclophosphamide 50mg/day) for nine months whereas nine patients in group B were directly shifted to phase III (only oral cyclophosphamide 50mg/day for nine months). Skin biopsy samples for DIF and blood samples for Dsg ELISA were taken again at randomisation and every three months for nine months thereafter.
Anti-Dsg 1 and 3 enzyme-linked immunosorbent assay
ELISA was performed on all the blood samples using the commercially available kits (MESACUP DSG- 1/ DSG- 3 ELISA TEST, MBL Co. Ltd, Nagoya, Japan). ELISA indices >20 were considered positive. Fall in ELISA indices from baseline to end of phase I was calculated. After randomization, fall in the ELISA indices was compared between the two groups every three months. A rise in the anti-Dsg antibody levels of 50% of ELISA indices between two three-monthly samples was defined as a relapse.
Direct immunofluorescence
DIF, 3-mm punch biopsy specimen transported in Michel′s medium were snap frozen and stained with Fluorescein Isothiocyanate-conjugated antisera to human IgG, IgM, IgA, C3 and fibrin. Slides were viewed under a Zeiss fluorescence microscope. All DIF slides were observed by two separate investigators. In DIF grading, 1+ denoted low positivity, 2+ denoted moderate positivity and 3+ and 4+ denoted high positivity.
Statistical analysis
Statistical analysis was performed using SPSS for windows. Continuous data was analyzed using the unpaired t-test. Nominal data was analyzed using chi-square test. Baseline data was analyzed using Student′s t-test. The significance of difference in the mean fall in ELISA index values in phase I was analyzed using paired t-test. The difference in the periodic DIF between the two groups was calculated using the chi-square test. Logistic regression method was used to investigate the relationship between continuous variables. Two-sided P values <0.05 were considered statistically significant.
Results
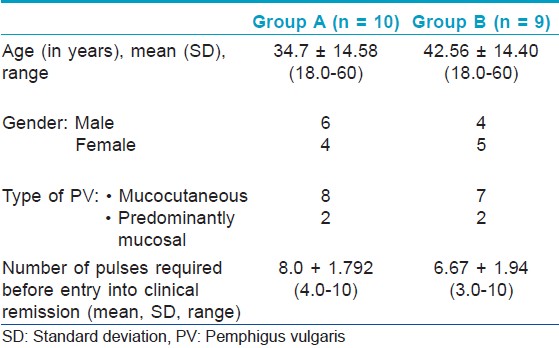
Out of 19 randomized patients, all completed the study. Both groups were comparable in terms of their baseline characteristics [Table - 1].
Phase I
The mean number of DCPs required in phase I was 6.5 (range 3-10).There was a significant fall in the mean Dsg 1 ELISA index (p=0.01) and Dsg 3 ELISA index (p=0.01) at the end of phase I, compared to baseline. Before treatment, 17 patients (89.5%) had strongly-positive DIF (3+,4+) whereas two patients (10.5%) had weakly-positive DIF (2+). At end of phase I, 1 patient (5.3%) had strongly positive DIF (3+), whereas 12 (63.2%) had weakly-positive DIF (2+,1+) and 6 (31.6%) had a negative DIF.
Trends in phase II phase
Anti-Dsg 1 enzyme-linked immunosorbent assay
At entry into phase II, the mean ± SD Dsg1 ELISA index of Group A was 38.28 ± 48.12 and 10.38 ± 9.97 in Group B. The mean Dsg 1 ELISA index showed a decreasing trend throughout phase II [Figure - 2]a.
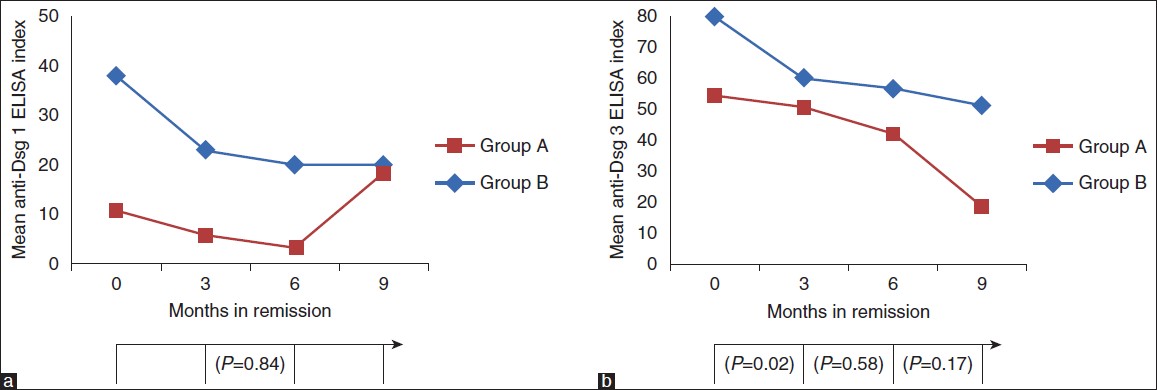 |
| Figure 2: Trends in the periodic Dsg ELISAs. (a) Dsg1 ELISA (b) Dsg3 ELISA. The P values represent the significance of the fall in the ELISA indices every 3 months |
Anti-Dsg 3 enzyme-linked immunosorbent assay
At entry into phase II, mean ± SD Dsg3 ELISA index was 80.14 ± 32.02 in Group A and 54.43 ± 47.22 in Group B. The mean Dsg 3 ELISA index showed a decreasing trend throughout phase II [Figure - 2]b.
Trends in the periodic direct immunofluorescence (DIF)
Being a semi-quantitative test, periodic mean of the DIF could not be calculated hence the percentage of patients with a negative DIF is presented separately [Figure - 3].
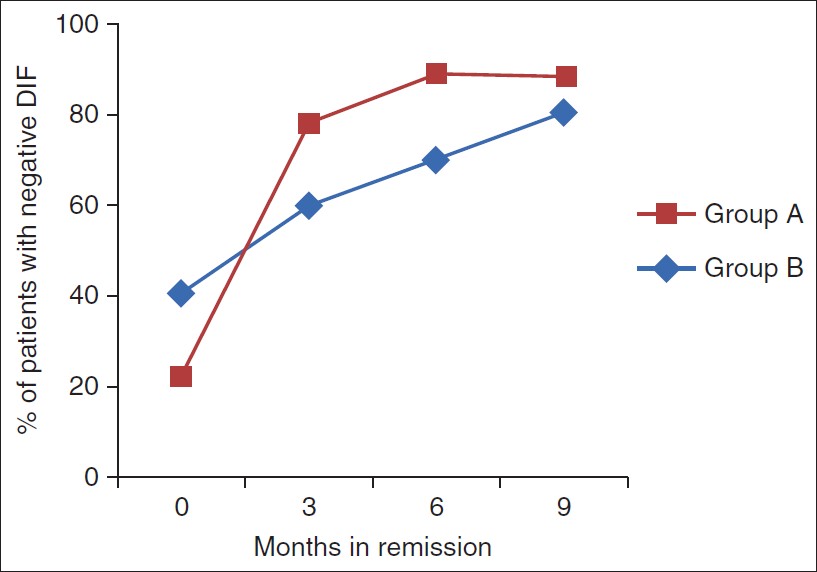 |
| Figure 3: Percentage of patients with negative DIF as assessed every 3 months during the phase II |
Incidence of relapse
According to the consensus statement on definitions of disease, end points and therapeutic response for pemphigus, [4] relapse is defined as the appearance of three or more new lesions in a month that do not heal spontaneously within one week, or the extension of established lesions, in a patient who has achieved disease control. By the end of nine months, one patient in each group had relapsed. Thus the incidence of relapse in Group A was 10% (1 of 10), and in Group B was 11.1% (one of nine). The patient in Group A was a case of mucosal-dominant type PV; he developed a mucosal relapse after being in remission for seven months. The patient in Group B was a case of muco-cutaneous PV who developed a cutaneous relapse after eight months of clinical remission. In both cases, the relapsed disease was less severe than the initial disease.
Sensitivity, specificity and predictive values of DIF and ELISA in detecting a relapse
In both patients who relapsed, DIF had become positive before the relapse. Keeping the criteria for relapse as DIF positivity after previous negativity, or an increase in the DIF grading from the previous reading, the sensitivity, specificity and positive predictive value for DIF in detecting a mucosal relapse was 100%, 88.9 % and 50%, respectively. For detecting a cutaneous relapse, the sensitivity, specificity and positive predictive value of DIF was 100% each, respectively.
As for Dsg ELISA, a slight modification in the criteria for relapse used by Abasq et al,[5] was used, which defined a rise in anti-Dsg antibodies as an increase of at least 50% of ELISA values between samples collected at relapse and those during the six month period before the relapse; here the three months period was used. For Dsg1 ELISA, the sensitivity, specificity and positive predictive value in detecting a cutaneous relapse in Group B was 100% each. However the Dsg3 indices continued to be negative in this patient during the entire study period. In Group A, one patient had a mucosal relapse, but his Dsg3 indices did not increase during this time, hence the sensitivity, specificity and positive predictive values could not be calculated
Discussion
We conducted this prospective randomized study to study the necessity of DCPs in phase II of the DCP regimen. All newly-diagnosed PV patients received DCPs until they were in clinical remission. In spite of being clinically disease-free, patients currently receive nine additional DCPs. This requires monthly hospital admission for at least three days and monitoring during pulse administration. The current practice is thus based entirely on the clinical course of the disease. We sought to study the immunological course of patients on this regimen.
DCP regimen was conceived by Pasricha and Ramji in 1984 after its success in a patient of Reiter′s disease. [6] This regimen revolutionized pemphigus treatment in India because of its efficacy, low cost of therapy and considerably less side effects of pulsed steroids compared to conventional steroids. [7] The major drawbacks of this regimen are its duration and lack of immunological monitoring. In a study to assess the long-term efficacy of DCP therapy, we previously observed that the time taken for completion of phase I was 4.2 months (range: 2-8 months), while time to completion of phase II and III was 24 months (range: 20-32 months). [8] In the present study the average number of pulses required in phase I was 6.5 (range 3-10).
The p value of the fall in ELISA index of both Dsg1 and Dsg3 before and after phase I was statistically significant (p=0.01). Subsequently however there was no statistically significant difference in the three-monthly measured ELISA index of Dsg1 and Dsg3 between the two groups. At randomization, 6 of 10 (60%) patients in Group A and all 9 (100%) in Group B had negative Dsg1 ELISA indices whereas 80% in Group A and 22.2% in Group B had negative Dsg3 ELISA indices. At the same time 40% of patients in Group A and 22.2% in Group B had a negative DIF, respectively. By the end of three months, 90% of Group A and all patients in Group B had negative Dsg1 ELISA indices, whereas 80% of Group A and 55.6% of Group B had negative Dsg3 ELISA indices. DIF was negative in 60% of patients in Group A and 77.8% of Group B. By the end of six months of remission, there was no further change in the antibody index profiles in either group compared to three months earlier. However a greater number of patients had a negative DIF compared to three months earlier, being 70% of Group A and 88.9% of Group B.
These findings are in accordance with the study conducted by Abasq et al, [5] in which they retrospectively reviewed 26 patients of PV and PF in clinical remission and serially measured their Dsg1 and Dsg3 ELISA titers, and the outcomes of these titers during the relapse of the disease. They observed that Dsg1 antibody titers reflect the course of disease, becoming negative with treatment, whereas Dsg3 titers decreased with treatment but still remained positive in spite of no clinical disease. They noted that persistent high levels (>100U/ml) or a rise (>50% from the value 6 months prior to occurrence of relapse) in Dsg1 titers heralded a clinical relapse. Similar findings were also reported by Kanwar et al[9] and Kwon et al,[10] in which the authors found poor correlation between Dsg 3 index values and disease activity. Mouquet et al,[11] observed that sera from PV patients in complete remission with persistently high anti-Dsg3 antibody levels no longer recognize the pathogenic N-terminal epitopes (EC1 and EC2) of Dsg3 that were targeted during the active phase of the disease. This explains that elevated anti-Dsg 3 ELISA titers do not reflect disease course or response to treatment.
Recently Saha et al,[12] reported a modification of Pasricha′s regimen using IV methylprednisolone instead of dexamethasone in combination with cyclophosphamide in 21 patients of refractory pemphigus. They used IIF and Dsg ELISA as outcome measures, and noted significant differences in both Dsg1 and Dsg3 titers, as well as IIF titers, between pre-pulse and one year post-pulse. David et al,[13] performed DIF on 24 patients in clinical remission with minimal doses of oral prednisolone and compared it with IIF; they performed two skin biopsies six months apart on each patient. They concluded that DIF is more sensitive than IIF, as small amounts of IgG deposits are detected in the epidermis by DIF, while IIF failed to detect similar amounts of circulating antibodies. Positive DIF in a state of clinical remission may have a significant prognostic value as an indicator of immunological activity of the disease; hence the treatment should be maintained.
One of the outcomes of the study was incidence of relapse at end of phase II. It was similar between the two groups, being 10% in Group A and 11.1% in Group B. The relapsed patient in Group A had mucosal dominant PV, which is usually recalcitrant to treatment. He developed a mucosal relapse while still receiving DCPs, revealing that the administration of additional DCPs did not prevent relapse; however, the type of pemphigus may be more predictive. In this patient the DIF was 2+ at the time of remission, but became 4+ before the occurrence of relapse by the end of six months. His Dsg3 ELISA indices remained positive but unaltered during relapse, whereas Dsg1 ELISA indices remained negative throughout [Table - 2]. These findings are interesting and again point that Dsg 3 index values may not correlate always with the disease activity.
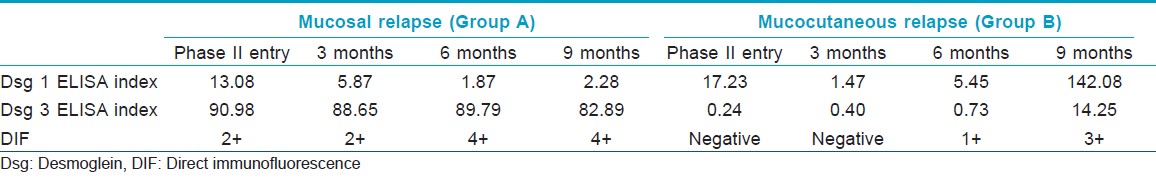
The patient who relapsed in Group B had mucocutaneous type PV and was non-compliant to treatment; she stopped taking her daily cyclophosphamide for two months before the occurrence of relapse. In this case also, DIF became positive before the relapse, being negative at the beginning and three months into phase II, 1+ by the end of six months, and 3+ by the end of nine months [Table - 2]. Her Dsg1 ELISA indices remained negative by the end of 6th month and became positive only by the end of nine months, whereas Dsg3 ELISA remained negative throughout. Thus DIF had a sensitivity and specificity of 100% in detecting a cutaneous relapse before its occurrence and had an advantage over Dsg ELISAs.
The major limitation of the present study is the small sample size and a short follow-up period of 9 months. Although our results suggested that additional 9 DCPs during phase II have no advantages, these results are preliminary and long term follow-up studies with larger sample size are required in the future to confirm our results.
In conclusion, although the DCP regimen can be used as a means of rapid control of PV, we found no difference in the clinical outcome between patients receiving 9 DCPs in phase II and patients shifted directly from phase I to III once clinical remission was achieved. Further large scale studies are required to assess if patients can be shifted directly from phase I to III and maintained only on oral cyclophosphamide. In this study by immunological and immuno-pathological investigations we found that Dsg ELISAs and DIF are reliable to guide further treatment of patients in clinical remission and in the prediction of a relapse. Though Dsg ELISAs have been shown to be quite specific and sensitive in the diagnosis and management of pemphigus patients their role during clinical remission has not been explored extensively. Our study has shown that in the two patients in whom there was a mucosal as well as cutaneous relapse, DIF became positive earlier than Dsg ELISA indices. However, our study had a limitation of small sample size with only two relapses and these findings also need confirmation in further studies with larger sample size. DIF combined with Dsg ELISAs may increase the sensitivity and specificity of detecting a cutaneous relapse in patients in clinical remission. It is wise to emphasize that this study is the first of its kind wherein both DIF and Dsg ELISAs have been used to monitor disease activity.
Acknowledgements
We gratefully appreciate the technical assistance of Ms. Ayumi Suzuki, Ms. Takako Ishikawa, and Ms. Sachiko Sakaguchi, and the secretarial work of Ms. Yasuko Nakayama, Ms. Emiko Hara, Ms. Hanako Tomita, Ms. Mihoko Ikeda, Ms. Kyoko Akashi, Ms. Nobuko Ishii, and Ms. Hanako Penner. We thank the patients for their participation. This study was supported by Grants-in-Aid for Scientific Research and Strategic Research Basis Formation Supporting Project from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Health and Labour Sciences Research Grants and grants for Research on Measures for Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan. The study was also supported by grants from the Uehara Memorial Foundation, Nakatomi Foundation, Kaibara Morikazu Medical Science Promotion Foundation, Japan Lydia O′Leary Memorial Foundation, Cosmetology Research Foundation, Japanese Dermatological Association (Shiseido Award), Fukuoka Foundation for Sound Health, and Galderma K.K. (Galderma Award).
| 1. |
Pasricha JS, Ramji G. Pulse therapy with dexamethasone and cyclophosphamide in pemphigus. Indian J Dermatol Venereol Leprol 1984;50:199-203.
[Google Scholar]
|
| 2. |
Amagai M, Komai A, Hashimoto T, Shirakata Y, Hashimoto K, Yamada T, et al. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol 1999;140:351-7.
[Google Scholar]
|
| 3. |
Kanwar AJ, De D . Pemphigus in India. Indian J Dermatol Venereol Leprol 2011;77:439-49.
[Google Scholar]
|
| 4. |
Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol 2008;58:1043-6.
[Google Scholar]
|
| 5. |
Abasq C, Mouquet H, Gilbert D, Tron F, Grassi V, Musette P, et al. ELISA testing of anti-desmoglein 1 and 3 antibodies in the management of pemphigus. Arch Dermatol 2009;145:529-35.
[Google Scholar]
|
| 6. |
Pasricha JS, Ramji G. Pulse Therapy with Dexamethasone in Reiter's Disease. Indian J Dermatol Venereol Leprol 1982;48:358-361.
[Google Scholar]
|
| 7. |
Pasricha JS, Thanzama J, Khan UK . Intermittent high-dose dexamethasone-cyclophosphamide therapy for pemphigus. Br J Dermatol 1988;119:73-7.
[Google Scholar]
|
| 8. |
Kanwar AJ, Kaur S, Thami GP. Long-term efficacy of dexamethasone-cyclophosphamide pulse therapy in pemphigus. Dermatology 2002;204:228-31.
[Google Scholar]
|
| 9. |
Kanwar AJ, Tsuruta D, Vinay K, Koga H, Ishii N, Dainichi T, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol 2011 [In Press].
[Google Scholar]
|
| 10. |
Kwon EJ, Yamagami J, Nishikawa T, Amagai M. Anti-desmoglein IgG autoantibodies in patients with pemphigus in remission. J Eur Acad Dermatol Venereol 2008;22:1070-5.
[Google Scholar]
|
| 11. |
Mouquet H, Musette P, Gougeon ML, Jacquot S, Lemercier B, Lim A, et al. B-cell depletion immunotherapy in pemphigus: Effects on cellular and humoral immune responses. J Invest Dermatol 2008;128:2859-69.
[Google Scholar]
|
| 12. |
Saha M, Powell AM, Bhogal B, Black MM, Groves RW . Pulsed intravenous cyclophosphamide and methylprednisolone therapy in refractory pemphigus. Br J Dermatol 2010;162:790-7.
[Google Scholar]
|
| 13. |
David M, Weissman-Katzenelson V, Ben-Chetrit A, Hazaz B, Ingber A, Sandbank M. The usefulness of immunofluorescent tests in pemphigus patients in clinical remission. Br J Dermatol 1989;120:391-5.
[Google Scholar]
|
Fulltext Views
2,804
PDF downloads
1,401





