Translate this page into:
Association between chronic renal disease and psoriasis risk in diabetes patients: A Korean population-based study
Corresponding author: Dr. Ji Hyun Lee, Department of Dermatology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea. yiji1@hanmail.net
-
Received: ,
Accepted: ,
How to cite this article: Yoo SA, Sayo MIA, Lee JH. Association between chronic renal disease and psoriasis risk in diabetes patients: A Korean population-based study. Indian J Dermatol Venereol Leprol. 2025;91:152-7. doi: 10.25259/IJDVL_669_2023
Abstract
Background
Several studies have reported that psoriasis has a positive correlation with type 2 diabetes mellitus (DM). Understanding the risk of psoriasis in diabetic patients is significant because it allows for early intervention and potential insights into the common pathways between the two conditions.
Objectives
We analysed the risk of psoriasis according to the estimated glomerular filtration rate (eGFR) and proteinuria level in DM patients using Korean population–based data.
Methods
This study was a retrospective cohort study using data collected from the country in the form of exploratory data analysis. A total of 927,234 participants diagnosed with DM were enrolled. Patients under the age of 20 with existing psoriasis or psoriasis developed within 1 year and missing data were excluded. The development of psoriasis was the primary outcome within a follow-up period of 7.83 ± 1.68 years.
Results
Of the 840,395 final participants, 28,010 (3.33%) patients developed psoriasis. In multivariate-adjusted Cox proportional hazards regression models, the DM patients with eGFR < 30 had a higher risk of psoriasis after adjustment (eGFR 60–90, hazard ratio [HR] 1 (Ref.); eGFR < 30, HR 1.173, 95% CI 1.089–1.264). In addition, there was an increased psoriatic risk of patients with DM and proteinuria after adjustment (negative, HR 1 (Ref.); 2+, HR 1.164, 95% CI 1.080–1.254; 3+, HR 1.433, 95% CI 1.273–1.613; 4+, HR 1.508, 95% CI 1.177–1.931).
Limitations
The severity of psoriasis was not measured since the occurrence of psoriasis was the outcome. Details of oral hypoglycaemic agents such as type and dose were not investigated.
Conclusion
This study showed that a decrease in eGFR and aggravation of proteinuria increase the risk of psoriasis in diabetic patients. Therefore, by using eGFR and proteinuria as predictive risk factors of psoriasis in DM patients, early and proactive treatment may play a vital role in managing diabetic patients.
Keywords
Diabetes
epidemiology
chronic
renal failure
proteinuria
psoriasis
Introduction
Psoriasis is a chronic inflammatory disease known to have various comorbidities.1–3 Two meta-analyses reported that the risk of diabetes mellitus (DM) increased in psoriatic patients with an odds ratio (OR) of 1.59 (95% CI 1.16–2.04) and 1.76 (95% CI 1.59–1.96), respectively. And one of them showed a dose effect (OR 2.10 in severe psoriasis, 95% CI 1.73–2.55).4,5A more recent meta-analysis and systematic review reported an OR of 1.69 (95% CI 1.51–1.89).6
Chronic kidney disease (CKD) is a complication of DM, diagnosed by a decrease in glomerular filtration rate (GFR) and symptomatic proteinuria.7,8 CKD and proteinuria have also been reported to be associated with psoriasis.1–3,9,10 A meta-analysis reported that patients with psoriasis had an increased CKD and end-stage renal disease (ESRD) risk with a pooled OR of 1.34 (95% CI 1.14–1.27) and 1.29 (95% CI 1.05–1.60), respectively.11 Another study reported that patients with psoriasis had an increased prevalence of pathologic albuminuria compared to controls (24% vs. 2%, P = 0.005), correlating with psoriasis severity (r = 0.458, P = 0.007).12
Although associations among psoriasis, DM, CKD, and proteinuria have been reported, there were no studies on the risk of psoriasis in diabetes patients with abnormal renal function. In this study, based on the fact that both psoriasis and diabetes are related to CKD and proteinuria, we investigated the risk of psoriasis according to eGFR and proteinuria level in diabetic patients using population-based data from the National Health Insurance Service (NHIS) of Korea.
Methods
Study population and data collection
This study was a retrospective cohort study using data collected from the country in the form of exploratory data analysis. The dataset was provided by the NHIS of South Korea and included information on the patient’s age, sex, income status, diagnosed diseases based on the International Classification of Disease 10 Clinical Modification (ICD-10-CM), medications, procedures, and medical examination results. Of note, the study population was recommended to undergo standard health examination every 2 years under the National Health Insurance Corporation.13
In the dataset, DM individuals were identified as either 1) using the ICD-10-CM code E11-14 and taking DM medication or 2) fasting glucose ≥126 mg/dL at the time of screening. DM patients with a confirmed diagnosis of DM during health screening in 2009 were enrolled in this study. Among 927,234 participants, those who were younger than 20 years (n = 85), diagnosed with DM and psoriasis before the study (n = 29,461), and diagnosed with psoriasis within 1 year (n = 11,085) were all excluded. In addition to this, 46,208 individuals with incomplete information were also excluded. Hence, the final study population was a complete data set of 840,395 participants [Figure 1]. This study was approved by the Ethics Committee of Seoul St. Mary’s Hospital, the Catholic University of Korea (KC22ZISI0406). The dataset was anonymised, and de-identified, and informed consent was waived by the Institutional Review Board of the Korean National Institute for Bioethics Policy. This was carried out according to the principles of the Declaration of Helsinki.
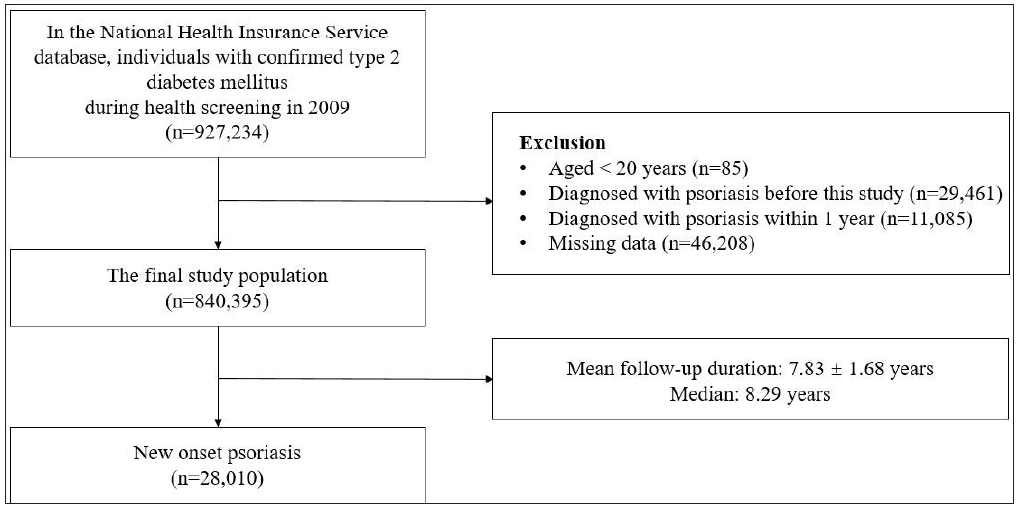
- Flow chart of this study.
Measurement and definition of covariates
Medical records included in this study were the past medical histories, blood pressure, weight, height, and laboratory test results for fasting glucose and estimated glomerular filtration rate. The body mass index (BMI) was calculated by weight in kilograms divided by the height in metres squared.14 Information on health-related behaviours was obtained using a standardised questionnaire. Smoking status was classified as a non-smoker, ex-smoker, and current smoker. Drinkers were classified as mild or heavy drinkers who consumed <30 g and ≥30 g of alcohol per day, respectively.15 Physical activity was an activity or exercise that make the individual more breathless than usual. Regular physical activity was taken to be either ≥30 minutes a day, five times a week for moderate physical activity, or ≥20 minutes a day, three times a week for strenuous physical activity. We defined the low income as individuals belonging to the lowest 25% of the income quartile.
Study design
To determine the association among psoriasis, CKD, and proteinuria in DM patients, the patients were divided into five subgroups according to eGFR levels and six subgroups according to proteinuria severity. In this study, CKD was defined as eGFR <60 ml/min/1.73 m2. During a follow-up period of 7.83 ± 1.68 years among the subgroups, patients newly diagnosed with psoriasis (ICD-10-CM code, L40) were identified.
Statistical analysis
The general, characteristics of DM patients are presented as means with standard deviations or numbers and percentages. The incidence possibility of psoriasis according to the presence of CKD and proteinuria in DM patients was obtained using Kaplan–Meier curves and the log-rank test was used to analyse differences between the groups. To analyse the presence and severity of CKD and proteinuria in association with psoriasis risk, multivariate-adjusted Cox proportional hazards regression models were used in two different ways: Model 1 was not adjusted for any variable. Model 2 was performed adjusting for age, sex, low income, smoking, drinking, regular physical activity, BMI, hypertension, dyslipidemia, fasting glucose, DM duration ≥5 years, and ≥3 oral hypoglycemic agents used. Model 3 was adjusted additionally for urine protein or eGFR. Variables in the models were chosen according to the clinical characteristics of developing psoriasis and the statistical significance of our study. Compared to controls (DM patients without CKD and proteinuria), hazard ratios (HRs) and 95% CIs were calculated. Statistical significance was defined as a two-sided P-value less than 0.05. All statistical analyses were performed using SAS software (ver. 9.4; SAS Institute, Cary, NC, USA) and R programming (version 3.1.0; The R Foundation for Statistical Computing, Vienna, Austria).16
Results
Baseline characteristics
The baseline characteristics of the patients according to the level of eGFR are summarised in Table 1. The baseline characteristics represented information from the 2009 medical examination. During the follow-up period with a mean of 7.83 ± 1.69 years, psoriasis was evident in 28,010 (3.33%) of the 840,395 DM patients.
| eGFR | p-value | |||||
|---|---|---|---|---|---|---|
| ≥90 | 60–90 | 45–60 | 30–45 | <30 | ||
| 278,622 | 455,490 | 73,548 | 13,236 | 19,499 | ||
| Age, years | 53.4 ± 11.3 | 58.0 ± 11.8 | 66.2 ± 9.7 | 68.2 ± 9.7 | 55.6 ± 12.3 | <.0001 |
| <40, n (%) | 31,814 (11.4) | 28,941 (6.4) | 644 (0.9) | 71 (0.5) | 1,928 (9.9) | |
| 40–64, n (%) | 206,250 (74.0) | 274,303 (60.2) | 27,280 (37.1) | 4,197 (31.7) | 12,652 (64.9) | |
| ≥65, n (%) | 40,558 (14.6) | 152,246 (33.4) | 45,624 (62.0) | 8,968 (67.8) | 4,919 (25.2) | |
| Male, n (%) | 182,394 (65.5) | 282,266 (62.0) | 32,251 (43.9) | 5,834 (44.1) | 13,472 (69.1) | <.0001 |
| Low income, n (%) | 59,106 (21.2) | 93,976 (20.6) | 15,091 (20.5) | 2,605 (19.7) | 2,545 (13.1) | <.0001 |
| Smoker, n (%) | <.0001 | |||||
| Non-smoker | 142,450 (51.1) | 254,752 (55.9) | 52,079 (70.8) | 9,513 (71.9) | 9,659 (49.5) | |
| Ex smoker | 48,598 (17.4) | 86,645 (19.0) | 11,219 (15.3) | 2,112 (16.0) | 4,701 (24.1) | |
| Current | 87,574 (31.4) | 114,093 (25.1) | 10,250 (13.9) | 1,611 (12.2) | 5,139 (26.4) | |
| Drinker, n (%) | <.0001 | |||||
| Non drinker | 140,437 (50.4) | 261,485 (57.4) | 55,053 (74.9) | 10,766 (81.3) | 10,621 (54.5) | |
| Mild drinker | 102,477 (36.8) | 150,863 (33.1) | 15,182 (20.6) | 2,059 (15.6) | 7,260 (37.2) | |
| Heavy drinker | 35,708 (12.8) | 43,142 (9.5) | 3,313 (4.5) | 411 (3.1) | 1,618 (8.3) | |
| Regular physical activity, n (%) | 59,615 (21.4) | 100,674 (22.1) | 15,022 (20.4) | 2,419 (18.3) | 4,616 (23.7) | <.0001 |
| BMI, kg/m2 | 24.9 ± 3.4 | 25.1 ± 3.2 | 25.2 ± 3.3 | 25.0 ± 3.4 | 24.7 ± 3.2 | <.0001 |
| <18.5 | 4,776 (1.7) | 5,870 (1.3) | 1,057 (1.4) | 220 (1.7) | 295 (1.5) | |
| <23 | 74,579 (26.8) | 109,387 (24.0) | 17,108 (23.3) | 3,310 (25.0) | 5,438 (27.9) | |
| <25 | 69,590 (25.0) | 116,988 (25.7) | 18,386 (25.0) | 3,296 (25.0) | 5,114 (26.2) | |
| <30 | 109,161 (39.2) | 191,785 (42.1) | 31,554 (42.9) | 5,397 (40.8) | 7,486 (38.4) | |
| ≥30 | 20,516 (7.4) | 31,460 (6.9) | 5,443 (7.4) | 1,013 (7.7) | 1,166 (6.0) | |
| Waist circumference, cm | 85.0 ± 8.6 | 85.6 ± 8.3 | 86.1 ± 8.6 | 86.6 ± 8.8 | 85.2 ± 8.2 | <.0001 |
| Hypertension, n (%) | 140,229 (50.3) | 261,649 (57.4) | 55,399 (75.3) | 11,364 (85.9) | 11,494 (59.0) | <.0001 |
| Dyslipidaemia | 102,814 (36.9) | 186,125 (40.9) | 36,877 (50.1) | 7,562 (57.1) | 8,112 (41.6) | <.0001 |
| Cardiovascular disease, n (%) | 11,079 (4.0) | 26,932 (5.9) | 8,332 (11.3) | 2,107 (15.9) | 1,452 (7.5) | <.0001 |
| Fasting glucose, mg/dL | 150.6 ± 50.4 | 145.5 ± 48.4 | 141.4 ± 51.5 | 141.6 ± 60.0 | 143.2 ± 48.5 | <.0001 |
| eGFR (mL/min/1.73 m2) | 110.2 ± 48.9 | 76.0 ± 8.0 | 54.6 ± 4.2 | 39.4 ± 4.0 | 8.9 ± 6.9 | <.0001 |
| DM duration ≥5 years, n (%) | 81,266 (29.2) | 148,105 (32.5) | 34,060 (46.3) | 8,083 (61.1) | 7,412 (38.0) | <.0001 |
| Insulin user, n (%) | 21,547 (7.7) | 37,359 (8.2) | 10,419 (14.2) | 3,606 (27.2) | 3,312 (17.0) | <.0001 |
| ≥3 Oral hypoglycaemic agent user, n (%) | 44,858 (16.1) | 74,371 (16.3) | 15,858 (21.6) | 3,402 (25.7) | 2,754 (14.1) | <.0001 |
| CKD, n (%) | - | - | 73,548 (100) | 13,236 (100) | 19,499 (100) | <.0001 |
| Urine protein positive, n (%) | 17,484 (6.3) | 31,733 (7.0) | 9,142 (12.4) | 3,483 (26.3) | 3,552 (18.2) | <.0001 |
Data are presented as means ± standard deviations or numbers and percentages.
BMI, body mass index; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease.
Association between eGFR and psoriasis in DM patients
As seen in Figure 2a, DM patients with eGFR<60 showed a higher incidence probability of psoriasis than those with eGFR≥60 (log-rank p < 0.0001). Psoriasis HR according to eGFR levels were assessed as seen in Table 2. Before adjustment, DM patients with CKD exhibited a higher risk of psoriasis than those without CKD [Table 2]. These results showed similar trends after adjustment for age, sex, low income, smoking, drinking, regular physical activity, BMI, hypertension, dyslipidemia, fasting glucose, DM duration ≥5 years, and ≥3 oral hypoglycemic agents used in Model 2. The lower the eGFR, the higher the HR of psoriasis in CKD patients [Table 2]. This tendency persisted after additionally adjusting the urine protein in Model 3 [Table 2]. In addition, the incidence rates of psoriasis showed similar tendencies with HRs [Figure 2b].
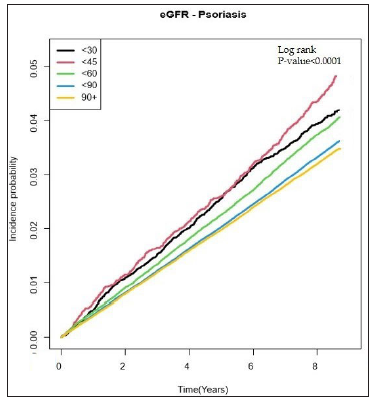
- Incidence probability of psoriasis according to the eGFR level in patients with diabetes mellitus.
| eGFR | n | Psoriasis, n (%) | Incidence rate (per 1000 person-years) | HR (95% C.I.) | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| ≥90 | 278,622 | 9,015 | 4.0 | 0.9 (0.9, 0.9) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) |
| 60–90 | 455,490 | 15,153 | 4.2 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 45–60 | 73,548 | 2,608 | 4.7 | 1.1 (1.0, 1.1) | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) |
| 30–45 | 13,236 | 501 | 5.5 | 1.3 (1.2, 1.4) | 1.1 (1.0, 1.2) | 1.0 (0.9, 1.1) |
| <30 | 19,499 | 733 | 4.9 | 1.1 (1.0, 1.2) | 1.1 (1.1, 1.2) | 1.1 (1.0, 1.2) |
| p-value | <0.0001 | <0.0001 | <0.0001 | |||
Model 1: Non-adjusted
Model 2: Age, sex, low income, smoking, drinking, regular physical activity, BMI, hypertension, dyslipidaemia, fasting glucose, DM duration ≥5 years, Insulin use, and oral hypoglycaemic agent ≥3 agents adjusted.
Model 3: Age, sex, low income, smoking, drinking, regular physical activity, BMI, hypertension, dyslipidaemia, fasting glucose, DM duration ≥5 years, Insulin use, oral hypoglycaemic agent ≥3 agents, and urine protein (positive vs. negative) adjusted.
eGFR estimated glomerular filtration rate; (HR hazard ratio, CI: Confidence interval)
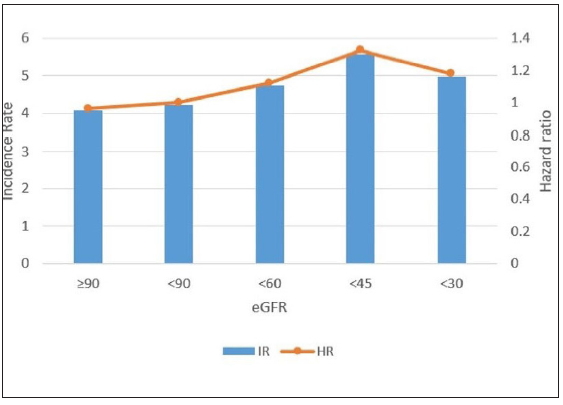
- Incidence rates and hazard ratios of psoriasis according to the eGFR level in patients with diabetes. (HR: Hazard ratios, IR: Incidence rates.)
Relationship between urine protein and psoriasis in DM patients
DM patients with proteinuria showed a higher incidence of psoriasis than those without proteinuria (log-rank p < 0.0001, [Figure 3a]). Psoriasis HR according to proteinuria levels were assessed and are presented in Table 3. Before adjustment, DM patients with proteinuria had a higher risk of psoriasis than those without proteinuria and the HR of psoriatic patients showed a positive correlation to the severity of proteinuria [Table 3]. This persisted after adjustment for age, sex, low income, smoking, drinking, regular physical activity, BMI, hypertension, dyslipidemia, fasting glucose, DM duration ≥5 years and ≥3 oral hypoglycemic agent use in Model 2 and even after additionally adjusting for eGFR in Model 3 [Table 3]. Further, the incidence rate of psoriasis also significantly increased as the proteinuria level increased [Figure 3b].
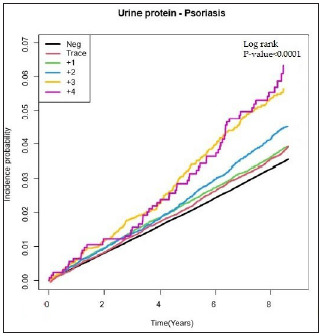
- Incidence probability of psoriasis according to the proteinuria level in patients with diabetes mellitus.
| Urine protein | Number | Psoriasis, n (%) | Incidence rate (per 1000 person-years) | HR (95% C.I.) | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| Negative | 743,503 | 24,428 | 4.1 | |||
| Trace | 31,498 | 1,115 | 4.5 | 1.0 (1.0, 1.1) | 1.060 (0.998, 1.125) | 1.060 (0.998, 1.125) |
| 1+ | 35,195 | 1,238 | 4.6 | 1.1 (1.0, 1.1) | 1.043 (0.985, 1.105) | 1.043 (0.985, 1.105) |
| 2+ | 18,189 | 714 | 5.3 | 1.2 (1.1, 1.3) | 1.164 (1.080, 1.254) | 1.164 (1.080, 1.254) |
| 3+ | 5,902 | 281 | 6.7 | 1.6 (1.4, 1.8) | 1.433 (1.273, 1.613) | 1.433 (1.273, 1.613) |
| 4+ | 1,267 | 63 | 7.1 | 1.7 (1.3, 2.2) | 1.508 (1.177, 1.931) | 1.508 (1.177, 1.931) |
| p-value | <.0001 | <.0001 | ||||
Model 1: Non-adjusted
Model 2: Age, sex, low income, smoking, drinking, regular physical activity, BMI, hypertension, dyslipidaemia, fasting glucose, DM duration ≥5 years, Insulin use, and oral hypoglycaemic agent ≥3 agents adjusted.
Model 3: Age, sex, low income, smoking, drinking, regular physical activity, BMI, hypertension, dyslipidaemia, fasting glucose, DM duration ≥5 years, Insulin use, oral hypoglycaemic agent ≥3 agents, eGFR adjusted.
HR: hazard ratio, CI: Confidence interval
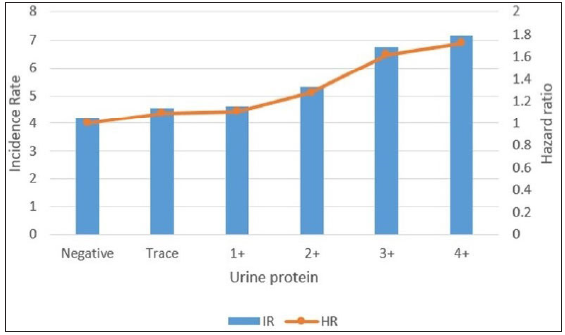
- Incidence rates and hazard ratios of psoriasis according to the proteinuria level in patients with diabetes. (IR: Incidence rates, HR: Hazard ratios.)
Discussion
Psoriasis is a chronic systemic inflammatory disease with a decreased quality of life and increased mortality rate for a variety of reasons.1,17–19 So far, the only study on the risk of psoriasis in diabetic patients was by Jacob et al. (2017).20 Comparing 72,148 diabetic patients with non-diabetic controls, they found that diabetic patients developed psoriasis more than controls (3.4% vs 2.8%). They hypothesised that psoriasis and diabetes share obesity and overweight as risk factors, that anxiety increases the risk of developing psoriasis in diabetic patients, and that skin infections may mediate the relationship between psoriasis and diabetes. On the other hand, we investigated the association between the two diseases from the new perspective of renal dysfunction, and found an association between renal dysfunction in diabetes and the development of psoriasis.
We identified that the risk and incidence rate of psoriasis increased as CKD and/or proteinuria progressed in diabetic patients. Although both CKD and proteinuria have been reported to be associated with psoriasis, whether they play a role in triggering psoriasis in diabetic patients is not yet known.9 The pathogenic mechanism shared between them is also not clearly established; however, several possible explanations are discussed below.
First, Th17 lymphocytes can induce inflammation in both psoriasis and kidney disease. Especially IL-17, one of the major pro-inflammatory factors in psoriasis, is known to play a crucial role in the development and progression of renal diseases such as glomerulonephritis, nephrotic syndrome, and diabetic nephropathy.11, 21
Second, both proteinuria and psoriasis reflect vascular damage. Proteinuria is the leakage of albumin from glomerular capillaries, resulting from damaged vascular endothelium. The incidence of peripheral vascular disease is higher in patients with psoriasis than those without.9 In addition to this, Creamer et al. (2002) observed systemic vascular hyperpermeability in patients with moderate to severe psoriasis, owing to elevated plasma VEGF concentration which could also induce hyperpermeability in the renal microvasculature.22
Third, oxidative stress contributes to the pathogenesis of both kidney disease and psoriasis. Duni et al. (2019) found that oxidative stress promotes inflammation through proinflammatory oxidised lipids or advanced oxidation protein products in diabetic nephropathy, IgA nephropathy, and polycystic kidney disease.23 Plenkowska et al. (2020) found that the total oxidative stress level increased in psoriasis, leading to the activation of signalling pathways and consequently to the activation of Th1 and Th17 cells.24
It is noteworthy that the group with eGFR ≥ 90 had a higher risk of psoriasis compared to the reference group (eGFR 60–90) after adjustment. The precise reason behind increased psoriasis risk in the eGFR ≥ 90 remains unclear; however, GFR exhibits favourable values during a ‘honeymoon period’ in the clinical course of DM due to glomerular hyperfiltration.25 It is possible that some individuals in the eGFR ≥ 90 group were classified as such due to glomerular hyperfiltration. Further, there is an ongoing debate on CKD staging, with some experts proposing the combination of stage 1 (GFR ≥ 90) and 2 (GFR 60–90).26 It is also worth noting that GFR can be influenced by a variety of factors including dehydration, protein intake, medications and others which may contribute to the consistently high risk of psoriasis in the GFR ≥ 90 groups.
Limitations
One limitation of this study is that the national database did not include information on the severity of psoriasis. Further research is needed to investigate the correlation between the progression of CKD/ESRD and psoriasis severity in diabetic patients. It is worth noting that psoriasis is typically diagnosed by a dermatologist and recorded in the data of NHIS of South Korea, but misclassification is possible. Additionally, this study did not investigate details of oral hypoglycemic agents such as type and dose. However, this is the first study to determine the relationship between the development of psoriasis, the progression of CKD, and the presence of proteinuria in diabetic patients based on the data obtained from a national population.
Conclusion
If psoriasis develops in patients with abnormal kidney function, treatment may be more difficult because of the limitations to the use of oral systemic immunosuppressants in them, and CKD itself increases the mortality in psoriasis patients.1,16 Therefore, according to the results of this study, proactive treatment to reduce the risk of psoriasis in diabetic patients by monitoring and early management of abnormal renal function is suggested; the eGFR as well as proteinuria may be important predictive factors therein.
Ethical approval
This study was approved by the Ethics Committee of Seoul St. Mary’s Hospital, the Catholic University of Korea (KC22ZISI0406).
Declaration of patient consent
Since this study used anonymised publicly available data, it was exempted from the IRB approval process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- All-cause and cause-specific mortality in psoriasis: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1332-43.
- [CrossRef] [PubMed] [Google Scholar]
- The global, regional, and national burden of psoriasis in 195 countries and territories, 1990 to 2017: A systematic analysis from the Global Burden of Disease Study 2017. J Am Acad Dermatol. 2021;84:46-52.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol. 2017;76:377-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Psoriasis and the risk of diabetes mellitus: A systematic review and meta-analysis. JAMA Dermatol. 2013;149:84-91.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis, psoriatic arthritis, and type 2 diabetes mellitus: A systematic review and meta-analysis. Br J Dermatol. 2013;169:783-93.
- [CrossRef] [PubMed] [Google Scholar]
- The association between psoriasis and diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13:1405-12.
- [CrossRef] [PubMed] [Google Scholar]
- Proteinuria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int. 2008;74:22-36.
- [CrossRef] [PubMed] [Google Scholar]
- Non-proteinuric diabetic nephropathy is the main cause of chronic kidney disease: Results of a general population survey in Spain. Diabetes Metab Syndr. 2017;11:S777-S81.
- [CrossRef] [PubMed] [Google Scholar]
- Proteinuria and psoriasis risk: A nationwide population-based study. J Clin Med. 2021;10
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease in psoriasis: A cohort study. J Dtsch Dermatol Ges. 2020;18:438-45.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis and risk of incident chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2018;50:1277-83.
- [CrossRef] [PubMed] [Google Scholar]
- The spectrum of renal abnormalities in patients with psoriasis. Int Urol Nephrol. 2012;44:509-14.
- [CrossRef] [PubMed] [Google Scholar]
- Cohort profile: The national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of socioeconomic status as a risk factor of pterygium using the Korean national health and nutrition examination survey 2010 to 2011: A STROBE-compliant article. Medicine (Baltimore). 2017;96:e6343.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cardioprotective effects of light-moderate consumption of alcohol: A review of putative mechanisms. Alcohol Alcohol. 2002;37:409-15.
- [CrossRef] [PubMed] [Google Scholar]
- Low hemoglobin levels and an increased risk of psoriasis in patients with chronic kidney disease. Sci Rep. 2021;11:14741.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quality of life of patients living with psoriasis: A qualitative study. BMC Dermatol. 2020;20:22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Psoriasis area severity index (PASI) and the dermatology life quality index (DLQI): The correlation between disease severity and psychological burden in patients treated with biological therapies. J EurAcad Dermatol Venereol. 2014;28:333-7.
- [CrossRef] [PubMed] [Google Scholar]
- Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182:840-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Psoriasis risk in patients with type 2 diabetes in German primary care practices. Prim Care Diabetes. 2017;11:52-6.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of end-stage renal disease in psoriatic patients: Real-world data from a nationwide population-based cohort study. Sci Rep. 2019;9:16581.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mediation of systemic vascular hyperpermeability in severe psoriasis by circulating vascular endothelial growth factor. Arch Dermatol. 2002;138:791-6.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress in the pathogenesis and evolution of chronic kidney disease: Untangling Ariadne’s thread. Int J Mol Sci. 2019;20
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int J Mol Sci. 2020;21
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Glomerular hyperfiltration in diabetes: Mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28:1023-39.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The definition, classification, and prognosis of chronic kidney disease: A KDIGO controversies conference report. Kidney Int. 2011;80:17-28.
- [CrossRef] [PubMed] [Google Scholar]







