Translate this page into:
Association between macrophage migration inhibitory factor-173G/C polymorphism and psoriasis risk: A meta-analysis
-
Received: ,
Accepted: ,
How to cite this article: Qi J, Zhang Y, Zhang L, Nie G. Association between macrophage migration inhibitory factor-173G/C polymorphism and psoriasis risk: A meta-analysis. Indian J Dermatol Venereol Leprol 2023;89:680-7
Abstract
Background
The association between macrophage migration inhibitory factor (MIF)-173G/C polymorphism and psoriasis risk has been reported in several studies with inconsistent conclusions.
Aims
This study aims to obtain a more convincing estimate of the relationship between the MIF-173G/C polymorphism and psoriasis risk.
Methods
Web of Science, EMBASE, PubMed, Wan Fang Database and Chinese National Knowledge Infrastructure (CNKI) were searched up to September 2021 and eligible studies were collected. The pooled odds ratios with 95% confidence intervals were calculated to estimate the effects of MIF-173G/C polymorphism on psoriasis risk under different genetic models. All analyses were conducted using the STATA12.0 software.
Results
A total of 1101 psoriasis cases and 1320 healthy controls from 6 relevant studies were included in this meta-analysis. Pooled analysis suggested that MIF-173G/C polymorphism was associated with increased psoriasis risk under the allelic model (C vs. G: odds ratio = 1.30, 95% confidence interval = 1.04–1.63, P = 0.020), heterozygous model (GC vs. GG: odds ratio = 1.53, 95% confidence interval = 1.05–2.22, P = 0.027) and dominant model (CC + GC vs. GG: odds ratio = 1.51, 95% confidence interval = 1.05–2.18, P = 0.027).
Limitation
Very few studies on the MIF-173G/C polymorphism in psoriasis have been reported till now, thus the number of studies included in the present meta-analysis was relatively small. Due to the number of studies being relatively small and the lack of raw data, stratified analysis by ethnicity or type of psoriasis was not carried out.
Conclusion
This meta-analysis demonstrated that MIF-173G/C polymorphism might be related to psoriasis risk. Carriers of the C allele and the GC genotype might have higher odds to present with psoriasis.
Keywords
Macrophage migration inhibitory factor (MIF)
meta-analysis
polymorphism
psoriasis
Plain Language Summary
MIF-173G/C polymorphism is the most researched single nucleotide polymorphism in the MIF gene affecting the transcriptional regulation and gene expression of MIF. The association between MIF-173G/C polymorphism and psoriasis risk has been reported in several studies with inconsistent conclusions. To obtain a more convincing estimate of the relationship between the MIF-173G/C polymorphism and psoriasis, we conducted a meta-analysis. Our results indicate that MIF-173G/C polymorphism might be related to psoriasis risk. Carriers of the C allele and the GC genotype might have higher odds to present with psoriasis.
Introduction
Psoriasis is a chronic and recurrent inflammatory disease which affects 1–3% the world’s population. 1 It primarily affects the elbows, knees, sacral region and scalp and is marked by well-defined erythematous–squamous plaques bilateral symmetric distribution. 2,3 Due to the chronic course and recurrent attacks, psoriasis can not only influence appearance but also affect the physical health and psychological status of patients. 4
The exact pathogenesis of psoriasis is not fully understood. Psoriasis was once thought to be a condition that solely affected the skin, but it is now widely recognized as a systemic inflammatory illness. 5 The “psoriatic march,” which refers to the presence of proinflammatory cytokines in patients’ blood as well as skin lesion, causes systemic inflammation. 6
MIF was identified in a guinea pig hypersensitivity experiment in 1966 and it was given that name because of its capacity to reduce macrophage mobility. 7 Subsequent research discovered that MIF can take part in a number of the host’s stress responses to microbial infection. These stress responses include activating macrophages, inhibiting migration and enhancing adhesion, phagocytosis and tumour-killing activities. 8–10 T cells, mononuclear macrophages, different haematopoietic cells, central and peripheral nerve cells, renal tubular epithelial cells, fat cells, vascular endothelial cells, liver cells, lung, prostate and testis all exhibit high levels of MIF expression. MIF may play a role in a number of physiological and pathological bodily functions, such as tissue damage and repair, immunological response, tumour genesis and inflammatory and immune responses. 11 Evidence now indicates that MIF plays a key role in the pathogenesis of psoriasis. 12
Single nucleotide polymorphism (SNP) mainly refers to the DNA sequence polymorphism caused by the variation of single nucleotide at the genome level. It is the most common human heritable variation, accounting for more than 90% of all known polymorphisms. It only involves the variation of a single base, which can be caused by the conversion, inversion, insertion or deletion of a base. However, the single nucleotide polymorphism usually mentioned does not include the latter two cases. The human MIF gene, located on chromosome 22q11.2, has a length of roughly 2,119 bp, three exons and two introns, and codes for 115 amino acids. The promoter of the MIF gene, which is situated at the binding location of the response element of transcription factor activator protein 4 (AP4), contains the polymorphism -173G/C. The MIF gene’s transcription level may be impacted by this polymorphism, which may influence the AP4 response element’s affinity. 13 In recent years, numerous studies have been conducted to investigate the relationship between MIF-173G/C polymorphism and susceptibility to psoriasis. 14–19 However, their conclusions were inconsistent or were contradictory. Therefore, we carried out a meta-analysis on the published relevant studies to clarify the correlation between MIF-173G/C polymorphism and psoriasis risk.
Materials and Methods
Search strategy
Web of Science, EMBASE, PubMed, Wan Fang Database and Chinese National Knowledge Infrastructure (CNKI) were independently searched by two authors till September 2021. The searched terms were “macrophage migration inhibitory factor or MIF,” “single-nucleotide polymorphism or SNP or polymorphism or variant” and “psoriatic or psoriasis.” All studies were limited to Chinese and English, and the references of the obtained literature were traced. The appropriate studies were located and thoroughly reviewed. Duplicates were first found, and then titles and abstracts were used to filter the remaining articles. If the abstracts piqued our curiosity, we read the whole texts.
Criteria for inclusion and exclusion
We ran a preliminary screening of all the literature based on titles and abstracts. Based on the inclusion criteria, we read the literature that was identified in the preliminary screen and acquired the final included literature. Studies were included if they met the following criteria: (1) were case-control studies; (2) were studies that documented the association between -173G/C polymorphism in the MIF gene and psoriasis susceptibility; (3) were studies that had available data to calculate 95% confidence interval for odds ratio. Correspondingly, the following studies were excluded for any of the following: (1) were duplications; (2) were not case-control studies; (3) were not related to the association between -173G/C polymorphism in the MIF gene and psoriasis susceptibility; (4) lacked the available data to calculate odds ratio and 95% confidence interval; (5) the genotype distributions of healthy controls were not consistent with the Hardy–Weinberg equilibrium (HWE).
Data extraction
Two reviewers independently completed the collection of the original literature record data. The following general information was extracted: first author, publication year, population, type of psoriasis, genotypic frequencies in cases and controls. If disagreements arose when extracting the data, they were settled by agreement reached by the third author.
Statistical analysis
The genotype distributions of healthy controls were tested by Pearson’s χ 2 test, and P < 0.05 was regarded as diverging from Hardy–Weinberg equilibrium. 20 Heterogeneity across studies was analysed by I 2 test and Q-test. 21 I 2 < 50% or P > 0.10 indicated no obvious heterogeneity across the original studies. Considering I2 can be unreliable in assessing heterogeneity when the number of studies is small as is the case here, random-effect model was applied to calculate odds ratios with corresponding 95% confidence intervals. The pooled odds ratios with corresponding 95% confidence intervals were calculated to estimate the effects of MIF-173G/C polymorphism on psoriasis susceptibly under different genetic models: allelic genetic model (C vs. G), homozygous genetic model (CC vs. GG), heterozygous genetic model (GC vs. GG) and dominant genetic model (CC + GC vs. GG) and recessive model genetic model (CC vs. GC + GG). The Z-test was used to assess the significance of the pooled odds ratio, and P < 0.05 was regarded as statistically significant. Sensitivity analysis, carried out by sequentially excluding a single individual study, was used to evaluate the robustness of results. We did not quantify the publication bias because it was inapplicable once the number of included papers was fewer than 10. All statistical tests were conducted using STATA 12.0 software (Stata Corporation, College Station, Texas).
Results
Characteristics of included literatures
According to the search strategy, 32 papers were initially detected. Through preliminary reading of the title and abstract, nine were excluded. After preliminary screening, the full text was read and Hardy–Weinberg equilibrium was examined in healthy controls. Finally, six papers were included in the present meta-analysis.
14–19
Figure 1 depicts the flow chart that details the study selection procedure. The publication year ranged from 2004 to 2021. The population including UK Caucasians, Chinese, Mexican-Mestizos, Turks and North Indians. The basic characteristics of each included study and genotypic frequencies are summarised in Tables 1 and 2.
HWE: Hardy–Weinberg equilibrium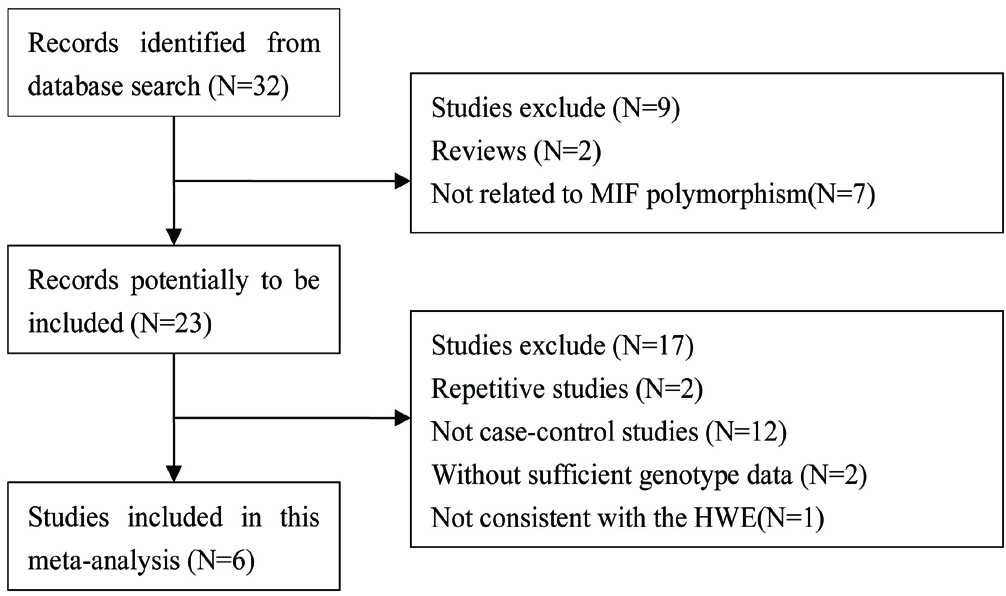
The flow chart of the selection of studies and specific reasons for exclusion from the meta-analysis
Study
Population
Sample size
Type of psoriasis
Case
Control
Donn RP (2004)
UK Caucasian
225
375
Plaque psoriasis
Wu J (2009)
Chinese
240
266
Plaque psoriasis
Morales-Zambrano R (2014)
Mexican–Mestizos
50
100
Psoriatic arthritis
Hernandez-Bello J (2021)
Mexican–Mestizos
224
232
Plaque psoriasis
Manav V (2021)
Turks
97
95
Including chronic local, chronic generalise, guttate, palmoplantar and inverse psoriasis
Chhabra S (2021)
North Indians
265
252
Plaque psoriasis
Study
Allele
Genotype
P
HWE
Case
Control
Case
Control
C
G
C
G
CC
GC
GG
CC
GC
GG
Donn RP (2004)
371
79
653
97
152
67
6
285
83
7
0.739
Wu J (2009)
368
112
421
111
137
94
9
163
95
8
0.184
Morales-Zambrano R (2014)
55
45
150
50
7
41
2
54
42
4
0.230
Hernandez-Bello J (2021)
313
135
352
112
107
99
18
135
82
15
0.595
Manav V (2021)
26
168
29
161
4
18
75
3
23
69
0.533
Chhabra S (2021)
401
129
376
128
144
113
8
139
98
15
0.677
Meta-analysis results
A total of 1101 psoriasis cases and 1320 healthy controls from 6 relevant studies were included in this meta-analysis. The single-nucleotide polymorphisms in the controls did not significantly deviate from the Hardy–Weinberg equilibrium (Table 2). Significant heterogeneities were observed in allelic genetic model (C vs. G), heterozygous genetic model (GC vs. GG) and dominant genetic model (CC + GC vs. GG) (I
2 > 50%). Pooled analysis suggested that MIF-173G/C polymorphism was associated with increased psoriasis risk in the allelic genetic model (C vs. G: odds ratio = 1.30, 95% confidence interval = 1.04–1.63, P = 0.020), heterozygous genetic model (GC vs. GG: odds ratio = 1.53, 95% confidence interval = 1.05–2.22, P = 0.027) and dominant genetic model (CC + GC vs. GG: odds ratio = 1.51, 95% confidence interval = 1.05–2.18, P = 0.027). No significant relation between them was found under the homozygous genetic model (CC vs. GG: odds ratio = 1.18, 95% confidence interval = 0.74–1.88, P = 0.486) and recessive genetic model (CC vs. GC + GG: odds ratio = 1.09, 95% confidence interval = 0.76–1.56, P = 0.641) (Table 3 and Figures 2a–2e). The sensitivity analysis was carried out by eliminating each study one by one and checking if the results changed. The overall results were not significantly altered, suggesting that the stability of this study is good.
Genetic model
Odds ratio
95% confidence interval
P-value
Heterogeneity
Effects model
I
2
P
C vs. G
1.30
1.04–1.63
0.020
57.9%
0.036
Random
CC vs. GG
1.18
0.74–1.88
0.486
15.5%
0.315
Random
GC vs. GG
1.53
1.05–2.22
0.027
71.3%
0.004
Random
CC + GC vs. GG
1.51
1.05–2.18
0.027
71.8%
0.003
Random
CC vs. GC + GG
1.09
0.76–1.56
0.641
0.0%
0.562
Random
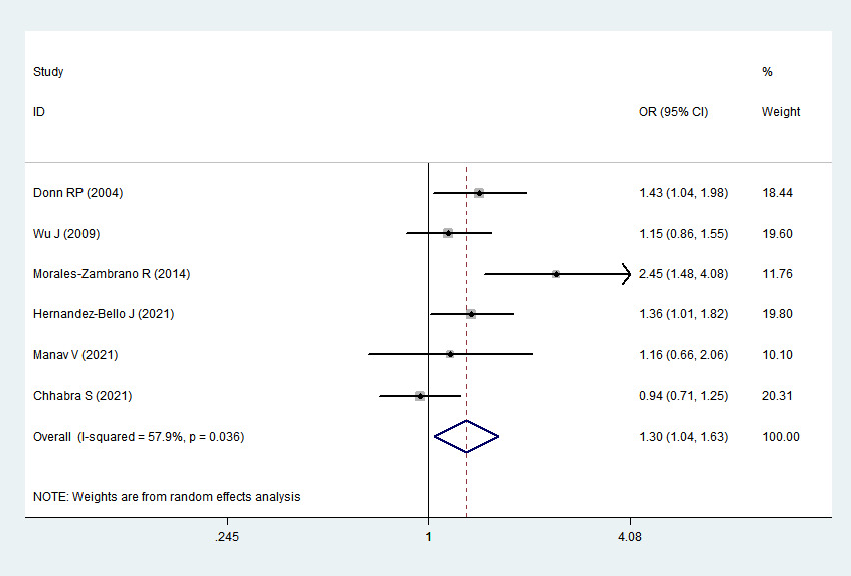
Association between MIF-173G/C gene polymorphism and psoriasis in allelic genetic model (C vs. G). CI, confidence interval; OR, odds ratio
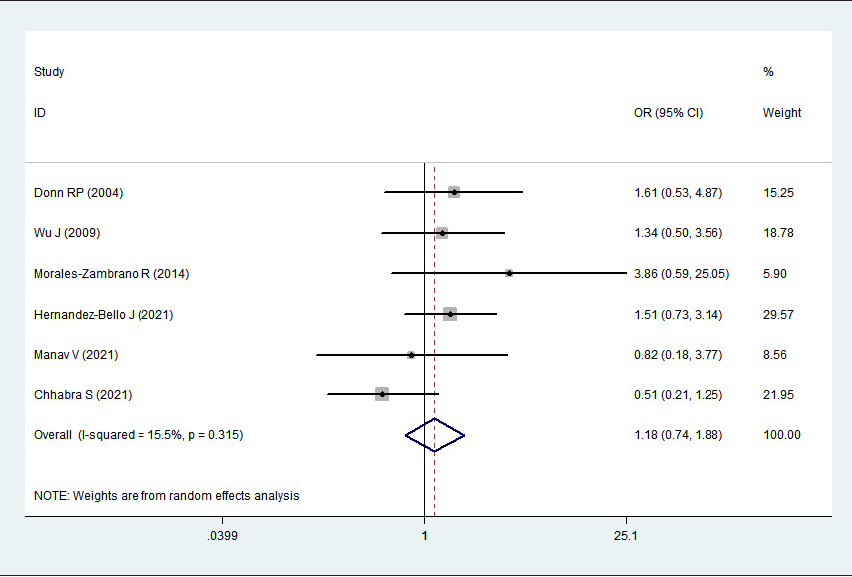
Association between MIF-173G/C gene polymorphism and psoriasis in homozygous genetic model (CC vs. GG). CI, confidence interval; OR, odds ratio
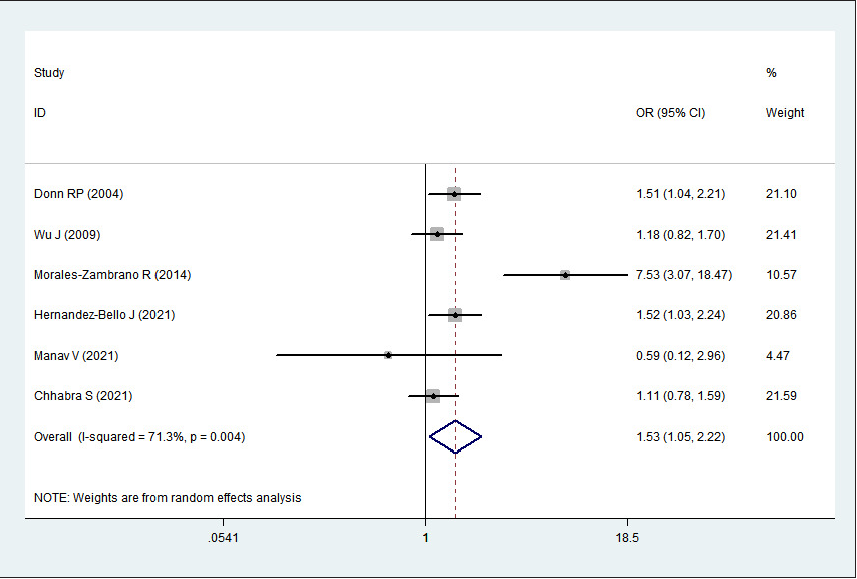
Association between MIF-173G/C gene polymorphism and psoriasis in heterozygous genetic model (GC vs. GG). CI, confidence interval; OR, odds ratio
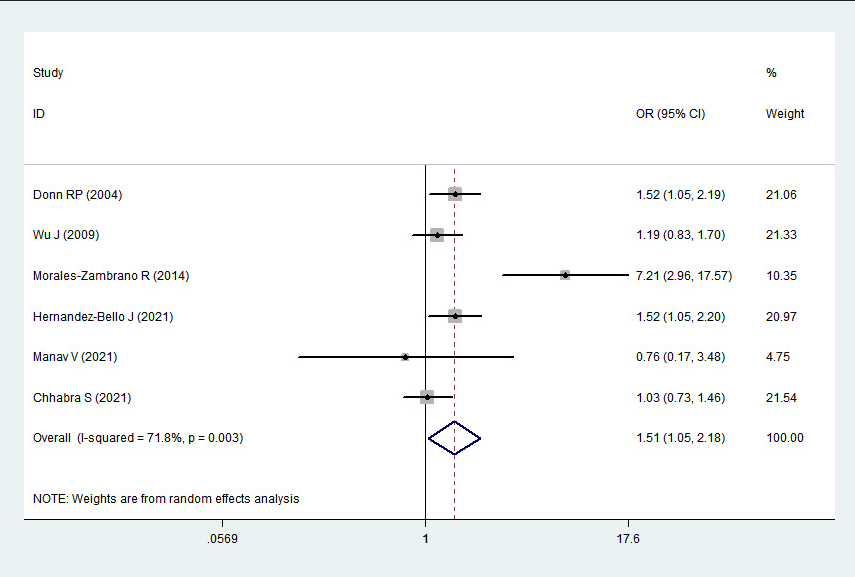
Association between MIF-173G/C gene polymorphism and psoriasis in dominant genetic model (CC + GC vs. GG). CI, confidence interval; OR, odds ratio
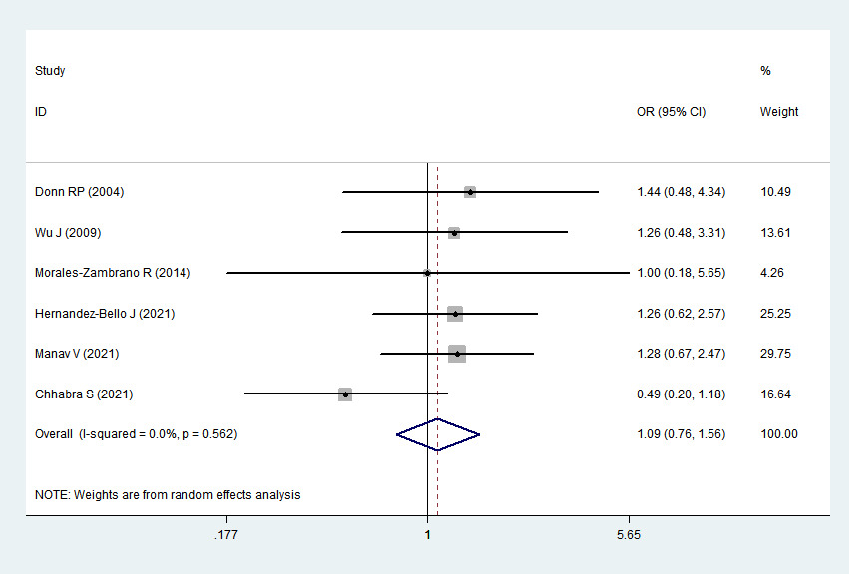
Association between MIF-173G/C gene polymorphism and psoriasis in recessive genetic model (CC vs. GC + GG). CI, confidence interval; OR, odds ratio
Discussion
Inflammation, keratinocyte hyper-proliferation, abnormal epidermal maturation and vascular changes are the major pathological symptoms of psoriasis. T-cell stimulation in the skin is caused by cellular immunity dysregulation, and the ensuing release of cytokines from T-cells boosts keratinocyte proliferation. 22 A confluence of immunologic disarray, susceptibility loci for psoriasis, autoantigens and several environmental variables may be the root cause of this complex disease. 23 Polymorphisms inside or close to several genes that encode cytokines have been linked to the pathomechanism of psoriasis, according to genome-wide association studies in patients with the condition. 24
MIF is well known as a crucial mediator to counter-regulate the suppressive effects of glucocorticoids on the immune system as well as a primary regulator of inflammation and a central upstream mediator of innate immune response. 25 A growing number of studies suggest that MIF polymorphisms may be associated with the risk of immune diseases. In patients with systemic lupus erythematosus, rheumatoid arthritis, etc., the promoter polymorphism at MIF-173G/C is linked to high levels of circulating MIF and higher disease susceptibility and severity. 25–28 The association between MIF-173G/C polymorphism and psoriasis has also been reported in several studies. According to Donn’s study of UK population, the MIF-173*C allele (GC + CC vs GG) was more prevalent in psoriasis patients than in controls, with odds ratio of 1.52 and 95% confidence interval of 1.05–2.19, respectively. 19 Similarly, the MIF-173*C allele was found to be associated with the susceptibility to psoriatic arthritis in Mexican–Mestizo population. 18 Another study in the Mexican–Mestizo population also found that individuals with the C allele (odds ratio = 1.34, 95% confidence interval = 1.005–1.807, P = 0.04) and the GC genotype (odds ratio = 1.51, 95% confidence interval = 1.026–2.228, P = 0.03) had a higher risk of developing plaque psoriasis. 15 However, there were no statistically significant variations in genotype or allele frequency with regard to the distribution of the MIF-173*G/C polymorphism between patients and controls in Chinese, 17 Turks 16 and North Indians 14 . The inconsistent findings may be explained by the weak statistical power of the investigations.
To obtain a more convincing estimate of the relationship between the MIF-173G/C polymorphism and psoriasis, we conducted a meta-analysis. This facilitates quantitative combined analysis and comprehensive evaluation of multiple case-control research results with the same research purpose, so as to improve the effectiveness of statistical test and solve the problem of inconsistent research results. It overcomes the shortcomings of small sample size and regional limitations. 29 To our knowledge, we were unable to find any previous meta-analysis examining the association between MIF-173G/C polymorphism and psoriasis. Our results indicate that MIF-173G/C polymorphism might be related to psoriasis risk. Carriers of the C allele and the GC genotype might have higher odds to present with psoriasis.
MIF appears to play a key role in the pathogenesis of psoriasis because it stimulates immune response and inflammation. 30,31 According to Shimizu’s study, serum MIF levels in psoriatic patients are higher than those in healthy controls. 12 However, MIF expression was found to be down-regulated in psoriatic cutaneous lesions. 12 They concluded that these discrepancies could be explained by the clinical stages of the patients, the precise location of the examined skin lesions and based on whether these were exposed to sunlight. They also interpreted this reduction as a counter-regulation in reaction to elevated serum levels. Further research on tissue samples is required to determine the precise function of MIF in the pathogenesis of psoriasis, primarily studies that control for clinical factors such disease activity, stage of the disease, ethnicity or treatment. All of them would clarifly MIF’s importance as a target for potential treatments for psoriasis or other skin conditions.
We did not quantify the publication bias since it was inapplicable once the number of included studies was fewer than 10. Sensitivity analysis is an important means to measure the quality of meta-analysis results. 32 The common reason affecting the stability of results is that the sample size of each included study is different. Large studies have a great impact on meta-analysis results. In this case, the results are easy to bias towards the conclusions of large sample study. Through sensitivity analysis, it was found that the results of this meta-analysis were stable.
Limitations
Only very few studies on the MIF-173G/C polymorphism in psoriasis have been reported till now, thus the number of literatures included in the present meta-analysis was relatively small. Therefore, the results should be interpreted cautiously. Due to the number of studies being small and the lack of raw data, stratified analysis by ethnicity or type of psoriasis was not carried out, and gene linkage effects were not assessed.
Conclusion
The results demonstrated that MIF-173G/C polymorphism might be a risk factor for psoriasis patients. Carriers of the C allele and the GC genotype might have higher odds to present with psoriasis. Further studies are needed to corroborate these findings in larger cohorts of multiethnic populations to clarify the precise function of MIF-173G/C polymorphism in psoriasis susceptibility.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-6.
- [CrossRef] [PubMed] [Google Scholar]
- Serum N-glycan profiling as a diagnostic biomarker for the identification and assessment of psoriasis. J Clin Lab Anal. 2021;35:e23711.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of psoriasis on quality of life in Kuwait. Int J Dermatol. 2006;45:418-24.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20:1475.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- “Inflammatory skin march” in atopic dermatitis and psoriasis. Inflamm Res. 2017;66:833-42.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80-2.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammation, infection and atherosclerosis: do antibacterials have a role in the therapy of coronary artery disease? Drugs. 2000;59:159-70.
- [CrossRef] [PubMed] [Google Scholar]
- A novel 5’-flanking region polymorphism of macrophage migration inhibitory factor is associated with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2001;44:1782-5.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage migration inhibitory factor (MIF) -173G/C promoter polymorphism influences upper gastrointestinal tract involvement and disease activity in patients with Crohn’s disease. Inflamm Bowel Dis. 2007;13:71-82.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 2010;23:257-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- High macrophage migration inhibitory factor (MIF) serum levels associated with extended psoriasis. J Invest Dermatol. 2001;116:989-90.
- [CrossRef] [PubMed] [Google Scholar]
- Association of MIF-173 gene polymorphism with inflammatory bowel disease in Chinese Han population. Cytokine. 2008;41:44-7.
- [CrossRef] [PubMed] [Google Scholar]
- Single-nucleotide polymorphism and haplotype analysis of macrophage migration inhibitory factor gene and its correlation with serum macrophage migration inhibitory factor levels in North Indian psoriatic patients with moderate disease severity: A cross-sectional study. Indian J Dermatol Venereol Leprol. 2023;89:247-53.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage migration inhibitory factor gene polymorphisms (SNP -173 G>C and STR-794 CATT5-8) confer risk of plaque psoriasis: A case-control study. J Clin Lab Anal. 2021;35:e23999.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Investigation of macrophage migration inhibitory factors and gene polymorphisms in patients with psoriasis. Turkderm. 2021;55:102-7.
- [Google Scholar]
- Association of MIF promoter polymorphisms with psoriasis in a Han population in northeastern China. J Dermatol Sci. 2009;53:212-5.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage migration inhibitory factor (MIF) promoter polymorphisms (-794 CATT5-8 and -173 G>C): association with MIF and TNFalpha in psoriatic arthritis. Int J Clin Exp Med. 2014;7:2605-14.
- [PubMed] [PubMed Central] [Google Scholar]
- Macrophage migration inhibitory factor gene polymorphism is associated with psoriasis. J Invest Dermatol. 2004;123:484-7.
- [CrossRef] [PubMed] [Google Scholar]
- Biased tests of association: comparisons of allele frequencies when departing from Hardy-Weinberg proportions. Am J Epidemiol. 1999;149:706-11.
- [CrossRef] [PubMed] [Google Scholar]
- Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Etiology and Pathogenesis of Psoriasis. Rheum Dis Clin North Am. 2015;41:665-75.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140:645-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cytokines in psoriasis. Cytokine. 2015;73:342-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The immunobiology of MIF: function, genetics and prospects for precision medicine. Nat Rev Rheumatol. 2019;15:427-37.
- [CrossRef] [PubMed] [Google Scholar]
- Dual effect of the macrophage migration inhibitory factor gene on the development and severity of human systemic lupus erythematosus. Arthritis Rheum. 2011;63:3942-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Macrophage migration inhibitory factor (MIF): genetic evidence for participation in early onset and early stage rheumatoid arthritis. Cytokine. 2013;61:759-65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Macrophage Migration Inhibitory Factor -173 G/C Polymorphism: A Global Meta-Analysis across the Disease Spectrum. Front Genet. 2018;9:55.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1:247-62.
- [CrossRef] [PubMed] [Google Scholar]
- Role of macrophage migration inhibitory factor (MIF) in the skin. J Dermatol Sci. 2005;37:65-73.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for a role of macrophage migration inhibitory factor in psoriatic skin disease. Br J Dermatol. 1999;141:1061-6.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis and sensitivity analysis for multi-arm trials with selection bias. Stat Med. 2011;30:1183-98.
- [CrossRef] [PubMed] [Google Scholar]






