Translate this page into:
Author's reply
2 Department of Dermatology, Bankura Sammilani Medical College, Bankura, West Bengal, India
Correspondence Address:
Amrita Sil
Department of Pharmacology, UCM Building (1st floor), 244 AJC Bose Road, IPGME and R, Kolkata-20, West Bengal
India
| How to cite this article: Sil A, Das N. Author's reply. Indian J Dermatol Venereol Leprol 2018;84:60-61 |
Sir,
We thank you for your critical appraisal of our study “Effectiveness and safety of levocetirizine 10 mg versus a combination of levocetirizine 5 mg and montelukast 10 mg in chronic urticaria resistant to levocetirizine 5 mg: A double-blind, randomized, controlled trial” by Sarkar et al.[1] We would like to clarify your doubts.
- Levocetirizine 10 mg one tablet in one group was compared to Levocetirizine 5 mg + Montelukast 10 mg one tablet in the other group. Thus, the participants of either group were given one tablet of either drug, keeping the blinding intact
- Clinical Trial Registry, India (CTRI) allows retrospective registration. However, ideally registration should be done prospectively. CTRI also takes some time to evaluate the proposal during registering, hence the time gap
- Both Urticaria Activity Score and Total Severity Score have been considered primary outcome measures, as mentioned in [Table - 2] of the article with results discussed accordingly. For objectively measuring the nonresponders, we had to consider Total Severity Score as it includes the count of the antihistamines used and because Urticaria Activity Score does not include the criteria of antihistamines used, since as physicians, we were concerned about the pill burden. Total Severity Score has been used previously in studies by Bajaj et al.[2] and Sil et al.[3]
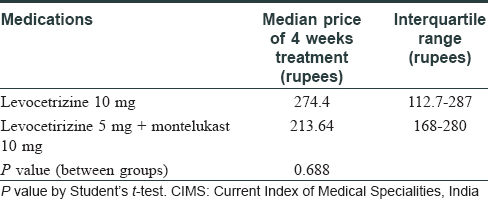
- The median price of levocetrizine 10 mg and levocetirizine 5 mg + montelukast 10 mg was compared from CIMS October–December, 2014 issue (at the time when the study was conducted). The cost of therapy was higher in levocetirizine 5 mg + montelukast 10 mg combination than levocetrizine 10 mg, as seen in [Table - 1] below. Also, in [Table - 2], when only the price of therapy of the trial medications (Levosiz 10 mg and Levosiz-M) was compared, the price of Levosiz M was 2.4 times more than Levosiz 10
- Because the study was designed as a superiority trial, the sample size was calculated based on detecting two-unit difference in Total Severity Score. We have mentioned that both the trial arms were “comparable in effectiveness.” Because the drugs were different molecules and not “me too” drug or drugs of the “same class,” we did not plan for an “equivalence” or “non-inferiority” trial.[4] Levocetirizine is an antihistamine whereas Montelukast is a leukotriene receptor antagonist. The article does not mention “equivalent” anywhere in the results or discussion. Moreover, the results (paragraph 3 of Results section) incorporate discussion on “between groups” analysis with the P values explicitly given in [Table - 2] of the article.

We hope we have clarified your doubts and would be eager to reply to your comments. The research team would like to thank you for your keen interest in our study.
Thanking you.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sarkar TK, Sil A, Pal S, Ghosh C, Das NK. Effectiveness and safety of levocetirizine 10 mg versus a combination of levocetirizine 5 mg and montelukast 10 mg in chronic urticaria resistant to levocetirizine 5 mg: A double-blind, randomized, controlled trial. Indian J Dermatol Venereol Leprol 2017;83:561-8.
[Google Scholar]
|
| 2. |
Bajaj AK, Saraswat A, Upadhyay A, Damisetty R, Dhar S. Autologous serum therapy in chronic urticaria: Old wine in a new bottle. Indian J Dermatol Venereol Leprol 2008;74:109-13.
[Google Scholar]
|
| 3. |
Sil A, Tripathi SK, Chaudhuri A, Das NK, Hazra A, Bagchi C, et al. Olopatadine versus levocetirizine in chronic urticaria: An observer-blind, randomized, controlled trial of effectiveness and safety. J Dermatolog Treat 2013;24:466-72.
[Google Scholar]
|
| 4. |
Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis 2008;66:150-4.
[Google Scholar]
|
Fulltext Views
2,180
PDF downloads
1,218





