Translate this page into:
Behcet's disease in India: A dermatological perspective
2 Department of Ophthalmology, University College of Medical Sciences and GTB Hospital, University of Delhi, Delhi, India
Correspondence Address:
Archana Singal
B-14, Law Apartments, Karkardooma, Delhi - 110 092
India
| How to cite this article: Singal A, Chhabra N, Pandhi D, Rohatgi J. Behcet's disease in India: A dermatological perspective. Indian J Dermatol Venereol Leprol 2013;79:199-204 |
Abstract
Background : Behcet's disease (BD) is a chronic, recurrent, multi-system inflammatory disorder involving mucocutaneous (MC), ocular, intestinal, articular, vascular, urogenital and neurologic systems. BD occurs with a high prevalence in the Mediterranean population. There is scarcity of clinical data on BD from India with only three case series in the last two decades. Aims: To study demographic profile, clinical manifestations and treatment outcome of patients with BD presenting to the dermatologic clinic in a tertiary hospital in north India. Methods: Prospective analysis of all patients diagnosed to have BD between 1997 to 2011. Result: Twenty nine patients were diagnosed to have BD. The disease had a female preponderance (M:F = 1:3.8) with a mean age of disease onset of 27.4 (range 16-61) years. The prevalence of various MC and systemic manifestations are as follows: oral aphthae (100%), genital aphthae (93.1%), erythema nodosum (62%), papulopustular and acneiform lesions (31%), articular involvement (68.9%), ocular involvement (31%) and gastrointestinal (GI) involvement (3.4%) . Pathergy test positivity was observed in 31%. The treatment comprised of colchicine (16/29 patients), dapsone (7/29), dapsone with pentoxiphylline (3/29), systemic steroid (2/29), systemic steroid with methotrexate (1/29). Colchicine was effective and well tolerated in all patients. Conclusion: The disease occurs in a much milder form in India and is primarily mucocutaneous and arthritic. A high index of suspicion in patients with MC lesions may result in early diagnosis, management and prevention of complications of BD. We suggest colchicine as an effective and safe therapeutic option for MC and joint involvement.Introduction
Behcet′s disease (BD) is a chronic, recurrent, multi-system inflammatory disorder of unknown aetiology, characterised by the triad of oral ulcers, genital ulcers and cutaneous lesions. This association was probably first recognised by Hippocrates, [1] but bears the name of Behcet after his detailed description of this illness in 1937. [2] The disease is now recognised to be a multi-system disorder involving mucocutaneous (MC), ocular, intestinal, articular, vascular, urogenital and neurologic systems. The diagnosis of BD is based on the identification of its typical clinical features as per the diagnostic criteria laid down by the International Study Group (ISG). [3] The disease has a worldwide distribution, being more common among populations with a higher prevalence of human leukocyte antigen (HLA) B5 and its split, HLA B51, in the Mediterranean basin, the Middle East and Far East. [4] Even though the prevalence of HLA B5 in north Indians (30%) is comparable to the Mediterranean (27%) [5] and the Japanese (36.9%) populations, [6] there are only two major studies from this region, one each from rheumatology and ophthalmology department. [7],[8] We present here the first case series from the dermatology clinic that is aimed to describe demographic and clinical profile of patients with BD and their response to treatment.
Methods
A prospective study was conducted on 29 consecutive patients, diagnosed to have BD in the department of dermatology, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi over a period of 15 years between 1997 to 2011. The diagnosis was based on the diagnostic criteria laid down by the ISG. [3]
A detailed history was elicited from each patient regarding the symptoms pertaining to MC and systemic involvement in the present episode and the frequency and number of past episodes if any, and was recorded in a pre-designed case record form. A careful clinical examination including examination of skin, mucosae (oral, genital and conjunctival) was carried out for evidence of any active or healed lesions. Patients were subjected to detailed ophthalmological examination for any evidence of active or healed uveitis and/or ulcers. Tuberculosis, sarcoidosis and syphilis were excluded based on clinical examination and investigations. Herpes simplex was excluded based on Tzanck smear from oral and/or genital ulcer and by performing Herpes simplex virus (HSV) serology for IgG and IgM antibodies in some subjects.
All patients underwent haematological investigations viz complete hemogram, liver function test (LFT), kidney function test (KFT), and Venereal Disease Research Laboratory (VDRL) along with urine microscopy. Mantoux test was done in all patients with 5 units of purified protein derivative (PPD) and reading recorded at 48-72 hours. Pathergy test was performed with a disposable 26 gauge needle injecting 0.1 ml normal saline at the flexor aspect of left forearm, approximately 2 inches below the elbow crease and read at 48 hours by one of the authors. Radiological evaluation included X-ray of the chest and clinically involved joints. For histopathological examination, biopsy specimens were taken from MC lesion or from the site of positive pathergy test. After informed consent, colchicine was prescribed as first line of treatment in 16 patients followed by dapsone in 10 and systemic steroids in 3 patients in addition to supportive management. The patients were followed up for a period of 6-12 months after the completion of treatment.
Results
29 patients were diagnosed to have BD during the study period of 15 years, which included 6 males (20.7%) and 23 females (79.3%), with a M:F ratio of 1:3.8. 16 patients (55.2%) belonged to the age group of 21-30 years. The mean age of disease onset was 27.4 years (range 16-61) and the mean duration of disease was 17 months (range 1-72). There was no family history of recurrent aphthosis or BD in any patient. Oral ulcers were present in all the patients (100%) [Figure - 1] followed by genital lesions in the form of active ulcers [Figure - 2] or healed scars in 27 patients (93.1%). 27 patients (93.1%) had cutaneous lesions, of which most common was erythema nodosum in 18 (62%) followed by papulopustular and acneiform lesions in 9 patients (31%).
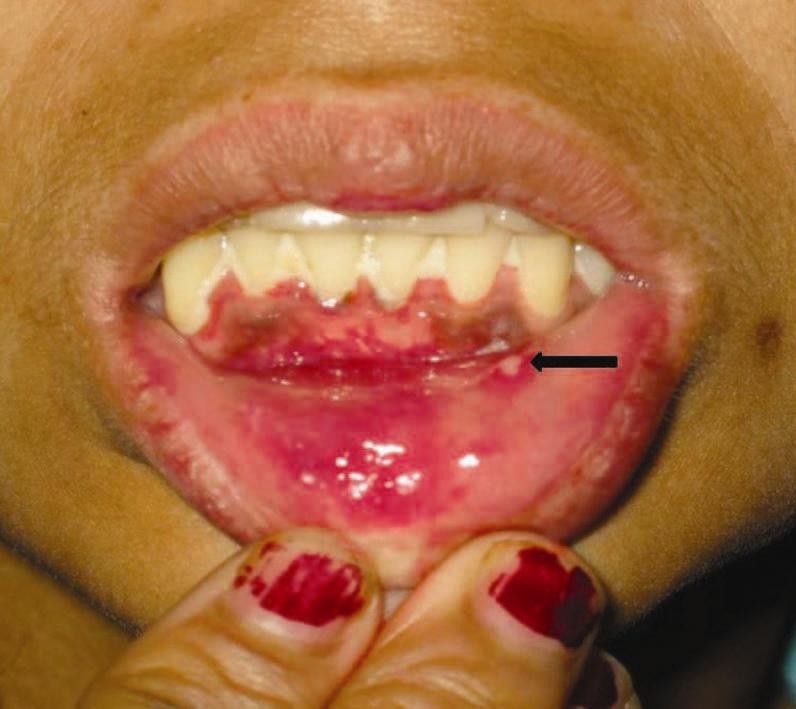 |
| Figure 1: Multiple confluent ulcers over lower gingival mucosa with single discrete ulcer over lower labial mucosa (arrow) |
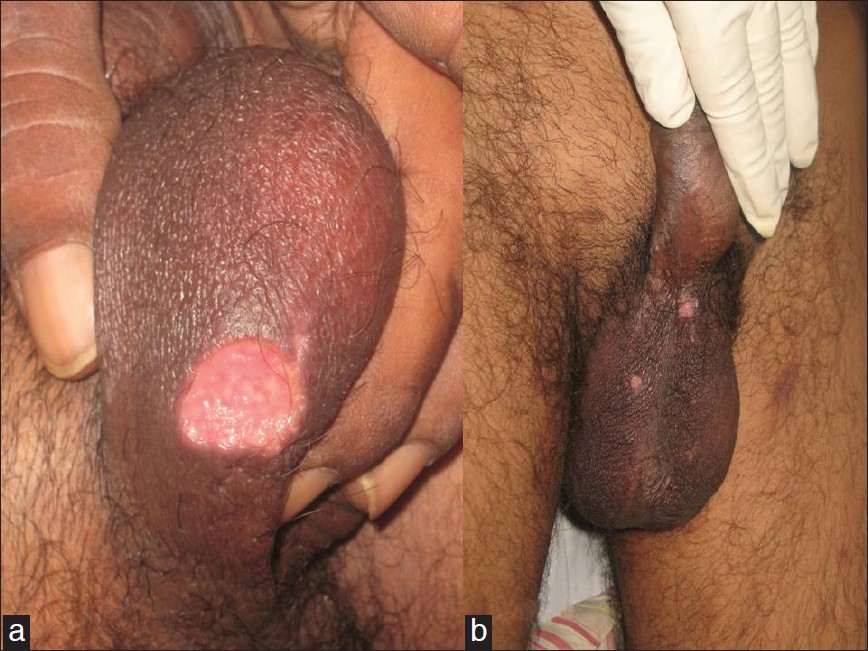 |
| Figure 2: Genital ulcer over ventral surface of scrotum (a) and over dorsal surface of scrotum (b) which is the most frequently involved site in male patients |
Ocular involvement was detected in 9 patients (31%) of which 5 had conjunctival ulcer and 2 each had iridocyclitis and uveitis. One female patient with uveitis had significant loss of vision (Right eye - 1/60 and left eye 6/24).
19 patients were febrile at the time of presentation (65.5%). Articular involvement was noted in 20 patients (68.9%) mostly affecting knee, wrist and ankle joints and manifested as swollen, painful joints with restriction of movements. 6 patients (20.7%) had tenosynovitis around wrist and ankle joint. Gastrointestinal (GI) involvement in the form of pain abdomen and hematemesis was seen in one patient (3.4%) who on further evaluation with upper GI endoscopy revealed multiple ulcers in the stomach and first part of duodenum.
A positive pathergy test was observed in 9 patients (31%).
Biopsy was done from the ulcer, skin lesions or positive pathergy site. Histopathology of erythema nodosum like lesions was consistent with lymphohistiocytic septal panniculitis in 10/18 patients and neutrophilic vascular reaction in 8/18 patients. Histopathology of papulopustular and acneiform lesions were consistent with neutrophilic vascular reaction in 5/9 patients while it showed perivascular lymphocytic infiltrate in 4/9 patients. Pathergy positive site biopsy showed neutrophilic vascular reaction in all the cases (9/29) [Figure - 3]. Raised ESR (>30mm) was observed in 26 patients (89.65%).
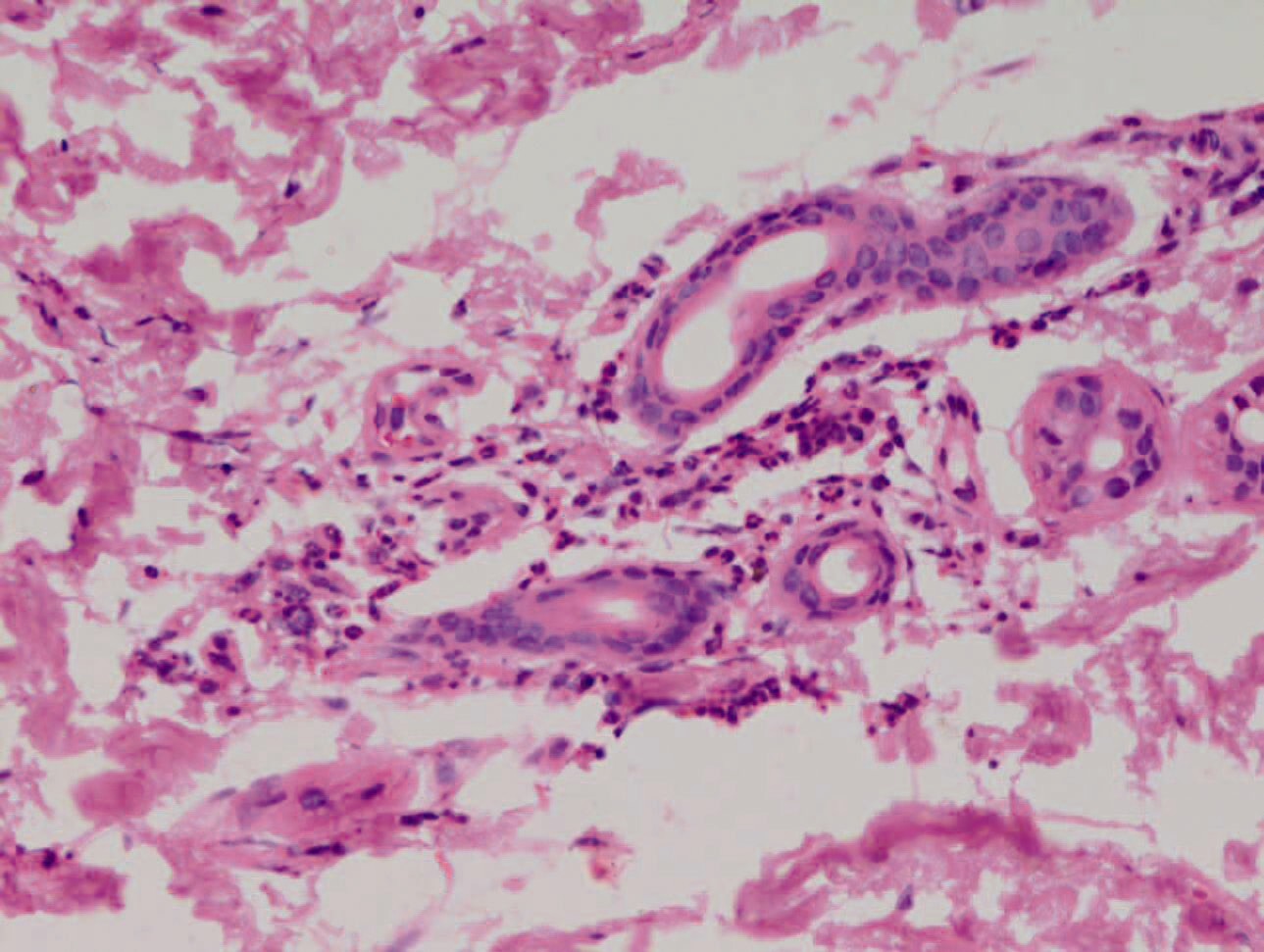 |
| Figure 3: Microphotograph showing perivascular neutrophilic infilterate with few extravasated RBCs in the dermis (H and E, × 400) |
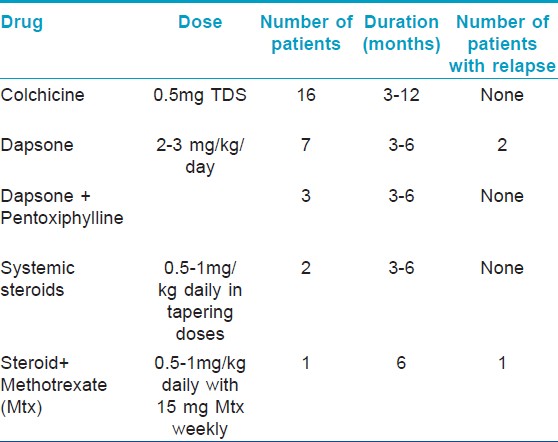
Colchicine was offered to significant number of the patients in this series. Of 29 patients, 16 (55.2%) received colchicine. Dapsone alone was given to 7 (24.1%) and in combination with pentoxiphylline in 3 patients (10.3%). Oral steroid in the form of prednisolone was prescribed to 2 patients (6.9%) who presented with uveitis. In one of the patient, methotrexate was given in addition in view of poor response to prednisolone alone and as a steroid sparing alternative. The dose and duration of treatment with various treatment regimes has been summarized in [Table - 1].
The outcome was favourable, with rapid and complete healing of the ulcers within 3-4 weeks and the treatment was continued for at least 3 months [Figure - 4]. The patients were followed up for about 6-12 months post treatment. In general, colchicine was well tolerated by all patients except for mild nausea and transient, self limiting diarrhoea in 4 patients. One of the female patients developed hemolytic anemia with dapsone 2 weeks after the treatment and was shifted to colchicine. 26/29 patients achieved complete clinical remission with no signs of relapse during the follow-up period of 6 months to 1 year. Three patients (10.3%) relapsed, two in the form of oral ulcers who had received dapsone and were switched on to colchicine while one had associated uveitis and was prescribed methotrexate with prednisolone.
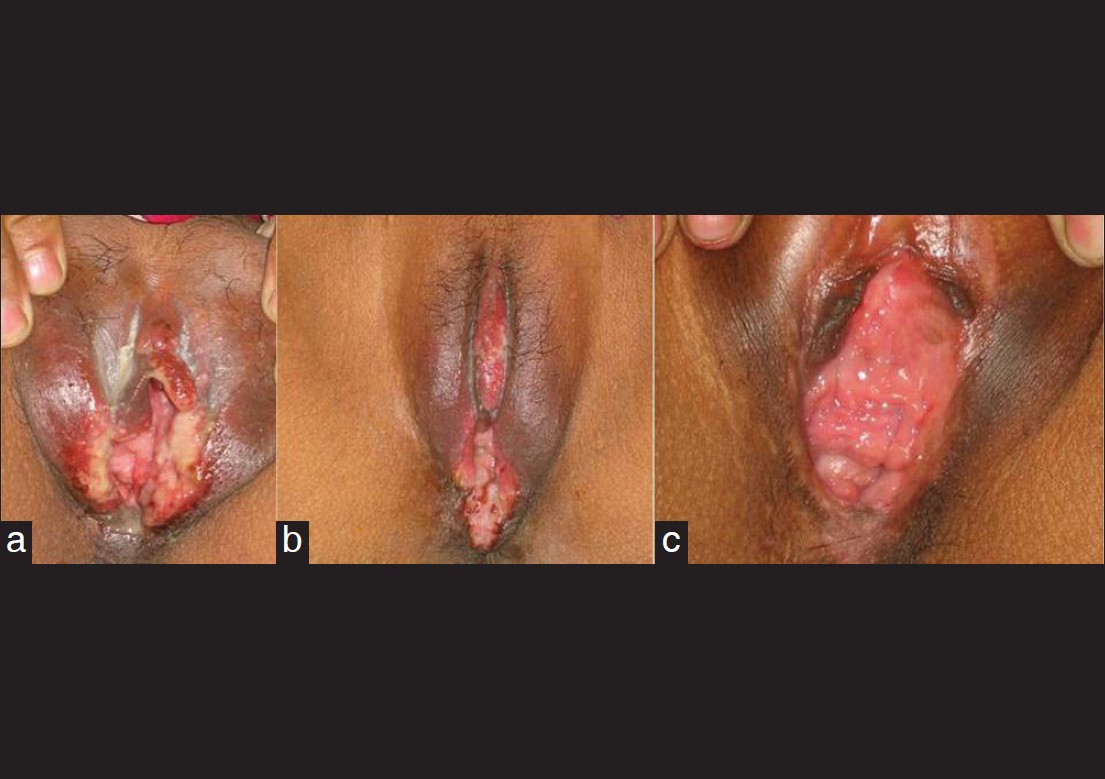 |
| Figure 4: Genital ulcer in a female patient at the time of presentation (a), improvement seen after 3 weeks (b), and after 3 months of treatment with colchicine (c) |
Discussion
Behcet′s disease (BD), also called Adamantiades-Behçet, is a systemic inflammatory disease presenting with recurrent oral aphthae, genital ulcer, cutaneous manifestations and uveitis. The exact etiology of BD is unknown, both genetic and environmental factors are thought to play a role in its pathogenesis. BD has been regarded as a Th1 type autoimmune disease, because of the association with HLA-B51 and hyper-reactivity against streptococcal antigen. The prevalence of HLA B5 in north Indians is high. [5] However, there are few reports of BD from India. [7],[8],[9],[10],[11] Only 2 major studies from north India exist; one from rheumatology department documenting 58 cases over a period of 16 years [7] and second is a recent retrospective study from uveitis clinic documenting 53 cases over 17 years. [8] From south India, a small series of 12 cases seen over 10 years has been published from the rheumatology department. [9] Ours is the first study from the department of dermatology reporting 29 patients in 15 years time and representing clinical spectrum of BD with special emphasis on MC involvement. The comparison of important parameters from the previous Indian studies has been tabulated [Table - 2].
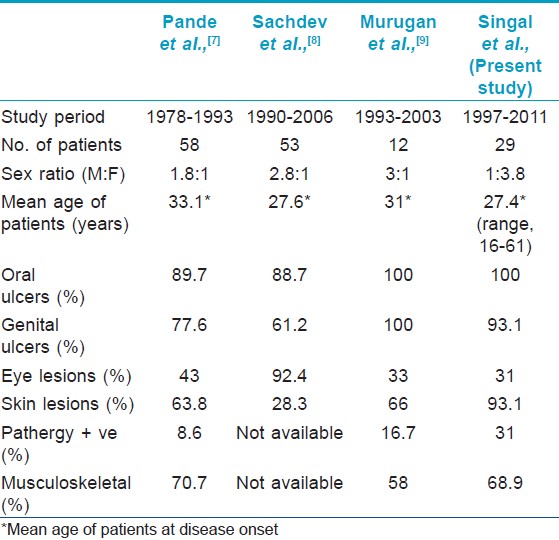
Behcet′s disease usually affects young adults between 20 to 40 years of age. [12] The mean age of disease onset in our study (27.4 years) was lower than that reported previously from India by Pande et al.,0 (33.1 yrs), [7] [Table - 2]. In contrast to previous Indian series [7],[8],[9] and in accordance with United States, Korea and Brazil, [13],[14],[15] we noted a female predominance (23/29) [Table - 2]. Male predominance has been reported by other countries also. [12],[16] Many authors have reported a higher frequency of genital ulcers in females as compared to males. [17],[18],[19] In the present study too, genital lesions were present in all female patients (23/23) and 4/6 of male patients.
Various MC manifestations in decreasing order of frequency in the present study are: oral ulcers, genital lesions and skin lesions. As ISG criteria (the diagnostic criteria used in the present study), do not permit diagnosis of BD in the absence of oral aphthae, [3] presence of oral aphthae was inevitable in all our patients. Other series from India have included patients with both complete as well as incomplete BD. Pande et al.,[7] documented oral ulcers as the most common finding (89.7%), however Sachdev et al., [8] documented ocular involvement more common than oral ulcers, which may be attributed to the recruitment of cases from uveitis clinic in their study [Table - 2]. Murugan et al. included only complete forms of BD, and thus oral ulcers were present in all the cases. [9]
Of the skin manifestations, erythema nodosum was most common followed by papulopustular lesions. According to published literature, there is a significantly higher frequency of erythema nodosum in females and of papulopustular eruptions in males. [17],[18],[19],[20] Higher ratio of females in our study may account for erythema nodosum as the most frequent cutaneous involvement.
Ocular involvement is one of the major causes of morbidity in BD. Occurrence of ocular lesions in BD vary from 50 to 85%, [14],[21] classical feature being acute anterior uveitis. [22] Incidence of ocular involvement at 31% in present series is similar to that of Gurler et al. from Turkey (28.9%). [23] Sachdev et al. reported panuveitis as the most common ocular involvement with complications in majority. [8] In our study, conjunctival ulcer was the most common presentation (5/29, 17.2%) followed by iridocyclitis (2/29, 0.7%) and uveitis (2/29, 0.7%), none had retinal involvement. Conjunctival ulcers are of rare occurrence [24],[25],[26],[27] with 5 out of 540 cases (0.9%) reported from Turkey and 4/ 152 (2.6%) cases from Japan. [25],[26] Surprisingly, we encountered high frequency of conjunctival ulcers in our study (17.2%) and reported this occurrence from India for the first time. [28] The necrotizing vascular lesions involving retina and optic nerve are often described in male patients. [29],[30] Female predominance in the present study may partly be held accountable.
The incidence of positive pathergy test was low (31%) in our study though it is highest amongst other Indian series [Table - 2]. The factors influencing pathergy test positivity include ethnicity, technique and test interpretation. It is most prevalent around the silk route. Males with BD in Turkish patients reportedly have a higher positive pathergy than females. [31] Use of a blunt, re-sterilised needle in Turkish patients may be responsible for high positivity rates. [32],[33] Practice of using disposable needles in West may partly explain the failure to demonstrate pathergy in Europe. [34] The interpretation of pathergy test is mainly clinical. A positive test invariably validates the diagnosis of BD with 98.4% specificity. [35] Recently, it has been suggested that thrombomodulin (TM) may serve as a marker for endothelial dysfunction in BD. [36] The authors found a strong correlation of high blood levels of TM with a positive pathergy test and suggested the measurement of TM as an alternative to the pathergy test.
Systemic involvement (musculo-skeletal, pulmonary, GI and neurological) was seen in a milder form. Arthropathy involving peripheral joints was the most common systemic feature. GI involvement was present in one male patient in the form of GI bleeding secondary to multiple gut ulcers and was referred to gastroenterology. Negligible systemic involvement in our study may be explained by the fact that the patients with primarily mucocutaneous complaints which is the earliest and most frequent feature of BD, presented to us. In different series, high systemic involvement, large vessel thrombosis, thrombophlebitis and pathergy positivity has been found in male patients, and in view of these data a more severe course in male patients may be expected. [17] Lesser number of males in our study might be another reason for the paucity of systemic features.
Treatment of BD remains challenging. Despite the wide spectrum of therapeutic agents used namely, systemic corticosteroids and immunosuppressive agents such as colchicine, azathioprine, cyclosporine, methotrexate, cyclophosphamide, sulphasalazine, dapsone, thalidomide, levimasole, chlorambucil, none of them has been found to be curative. No standard therapy has yet been established for the disease. The choice of treatment is based on the severity of clinical manifestations. Colchicine was used as a primary modality in our study. None of the patient had any significant side effect and no relapse was seen during follow up. In a randomized, double blind, crossover trial comprising of 169 BD patients without major organ involvement, Davatchi et al., reported significant improvement with colchicine in the overall disease activity, oral and genital ulcers, papulopustular lesions, and erythema nodosum. [37] Dapsone was used in our patients as an alternative to colchicine with good clinical response but relapse was seen in 2 out of 7 patients. Sharquie et al.,[38] in their double-blind, crossover study of 20 patients, reported significant reductions in the number, duration, and frequency of oral ulcers and number of genital ulcers with dapsone. This compound also showed a significant decrease in the frequency of skin lesions. Arthritis and epididymitis also responded significantly to dapsone, but its effect on arthralgia failed to reach the level of statistical significance. In our series, the indication for corticosteroid use and immunosuppressive (methotrexate) was for the control of uveitis.
In conclusion, despite the small number of BD patients recruited in our department, this study confirms that MC manifestations are hallmarks of the disease onset. Our findings corroborate the findings of Pande et al.,[7] that the disease occurs in a much milder form in India and is primarily MC and arthritic, other systemic features being less frequent. We emphasize that a high index of suspicion in a patient with MC lesions may result in early diagnosis, management and prevention of complications. A detailed ophthalmologic evaluation in all patients is recommended. The sensitivity of pathergy test as diagnostic criteria is reported low in Indian series, nonetheless the high specificity maintains its importance as a diagnostic criterion. In our experience, colchicine works well for the MC involvement and arthropathy in BD, it is well tolerated and associated with good safety profile and long term remission.
| 1. |
Feigenbaum A. Description of Behcet's Syndrome in the Hippocratic Third book of Endemic Diseases. Br J Ophthalmol 1956;40:355-7.
[Google Scholar]
|
| 2. |
Behcet H. As quoted by Duke Elders, Perkin ES. Inflammation of the uveal tract: Uveitis. In: Duke-Elders, editor. Vol. 9. System of Ophthalmology. London: S Henry Hampton; 1966. p. 358-73.
[Google Scholar]
|
| 3. |
International Study Group for Behcet's Disease. Criteria for Diagnosis of Behcet's Disease. Lancet 1990;335:1078-80.
[Google Scholar]
|
| 4. |
Ohno S, Ohguchi M, Hirosa S, Matsuda S, Wakisaka A, Aizawa M. Close association of HLA B51 with Behcet's Disease. Arch Ophthalmol. 1982;100:1455-8.
[Google Scholar]
|
| 5. |
Mehra NK, Taneja V, Kailash S, Vaidya MC. Distribution of HLA Antigens in a sample of North Indian Hindu Population. Tissue Antigens 1986;27:64-74.
[Google Scholar]
|
| 6. |
Yazici H, Akokan G, Yalein B, Mufatuoglu A. The high prevalence of HLA B5 in Behcet's Disease. Clin Exp Immunol 1977;30:259-61.
[Google Scholar]
|
| 7. |
Pande I, Uppal SS, Kailash S, Kumar A, Malaviya AN. Behcet's Disease in India: A Clinical, Immunological, immunogenetic and outcome study. Br J Rheum 1995;34:825-30.
[Google Scholar]
|
| 8. |
Sachdev N, Kapali N, Singh R, Gupta V, Gupta A. Spectrum of Behcet's disease in the Indian population. Int Ophthalmol 2009;29:495-501.
[Google Scholar]
|
| 9. |
Arulrajamurugan, Rajendran CP, Ravichandra R, Parthiban M, Rajeswari S, Rukmangatharajan S, et al. Clinical profile of patients with Behcet's disease. J Indian Rheumatol Assoc 2005;13:135-7.
[Google Scholar]
|
| 10. |
Singh RR, Malaviya AN. Behcet's Syndrome in North India.J Assoc Physicians India 1988;36:238.
[Google Scholar]
|
| 11. |
Kunjuraman G, Joseph PP, Philip J. Behcet's Syndrome: A report of 3 cases with review of literature. J Assoc Physicians India 1980;28:195-8.
[Google Scholar]
|
| 12. |
Benamour S, Bennis R, Amraui A. A study of 285 cases of Behçet's disease. In: O'Duffy JD, Kökmen E, editors. Behçet's Disease Basic and Clinical Aspects. New York: Marcel Dekker Inc. 1991. p. 259-67.
[Google Scholar]
|
| 13. |
Davis GL, Brissett D. Experiencing Behçet's Disease: A view from 245 patients. In: Godeau P, Wechsler B, editors. Behçet's Disease. Amsterdam: Elsevier Science Publishers; 1993. p. 211-4.
[Google Scholar]
|
| 14. |
Bang D, Yoon KH, Chung HG, Choi EH, Lee ES, Lee S. Epidemiological and clinical features of Behçet's Disease in Korea. Yonsei Med J 1997;38:428-36.
[Google Scholar]
|
| 15. |
Scherer MA, Vitral N, Orefice F. Clinical study of Behçet's Disease in Brazil. In: Godeau P, Wechsler B, editors. Behçet's Disease. Amsterdam: Elsevier Science Publishers; 1993. p. 181-4.
[Google Scholar]
|
| 16. |
Gharibdoost F, Davatchi F, Shahram F, Akbarian M, Chams C, Chams H, et al. Clinical Manifestations of Behçet's Disease in Iran Analysis of 2176 cases. In: Godeau P, Wechsler B, editors. Behçet's Disease. Amsterdam: Elsevier Science Publishers; 1993. p. 153-8.
[Google Scholar]
|
| 17. |
Tursen U, Gurler A, Boyvat A. Evaluation of clinical findings according to sex in 2313 Turkish patients with Behçet's disease. Int J Dermatol 2003;42:346-51.
[Google Scholar]
|
| 18. |
O'Neill TW, Rigby AS, McHugh S, Silman AJ, Barnes C. Regional Differences in clinical manifestations of Behçet's disease. In: Godeau P, Wechsler B, editors. Behçet's Disease. Amsterdam: Elsevier Science Publishers; 1993. p. 159-63.
[Google Scholar]
|
| 19. |
Alli N, Gur G, Yalcin B, Hayran M. Patient characteristics in Behçet disease: A retrospective analysis of 213 Turkish patients during 2001-4.Am J Clin Dermatol 2009;10:411-8.
[Google Scholar]
|
| 20. |
Zouboulis ChC, Djawari D, Kirch W. Adamatiades-Behçet's Disease in Germany. In: Godeau P, Wechsler B, editors. Behçet's Disease. Amsterdam: Elsevier. Science Publishers; 1993. p. 193-6.
[Google Scholar]
|
| 21. |
Nazzaro P. Cutaneous manifestations of Behcet's Disease. Clinical and histopathological findings. In: Monacelli M, Nazzaro P, editors. Rome: Int Symp on Behcet's disease; 1965. Basel: S Karger; 1966. p. 15-41.
[Google Scholar]
|
| 22. |
Barra C, Belfort R, Abreu MT, Kim MK, Martins MC, Petrilli AM. Behcet's Disease in Brazil: A review of 49 cases with emphasis on ophthalmic manifestation. Jpn J Ophthalmol 1991;35:339-46.
[Google Scholar]
|
| 23. |
Gurler A, Boyvat A, Tursen U. Clinical manifestations of Behcet's disease: An analysis of 2147 patients. Yonsei Med J 1997;38:423-7.
[Google Scholar]
|
| 24. |
Ouertani A, Lasram L, Mili I. Behcet's Disease disclosed by ocular conjunctival aphthous ulcer. Apropos of a case. J Fr Ophthalmol 1992;15:131-2.
[Google Scholar]
|
| 25. |
Pazarli H, Ozyagzan Y, Bahcecioglu H, Yazici H, Hattat N, Yurdakal S, et al. Ocular involvement in Behcet's Syndrome in Turkey. In: Lehner T, Barnes CG, editors. Recent Advances in Behcet's Disease. London, New York: Royal Society of Medicine Services; 1986. p. 267-8.
[Google Scholar]
|
| 26. |
Matsuo T, Itami M, Nakagawa H, Nagayama M. The Incidence and Pathology of Conjunctival Ulceration in Behcet's Syndrome. Br J Ophthalmol 2002;86:140-3.
[Google Scholar]
|
| 27. |
Otmani F, Brouri M, Brouri H, Abbes L, Berrah A. Conjunctival Aphthosis; an unusual site in Behcet's Disease. Presse Med 1995;24:1708.
[Google Scholar]
|
| 28. |
Rohatgi J, Singal A. Ocular manifestations of behcet's disease in Indian patients. Indian J Ophthalmol 2003;51:309-13.
[Google Scholar]
|
| 29. |
Ames PR, Steuer A, Pap A, Denman AM. Thrombosis in Behcet's disease: A retrospective survey from a single UK centre. Rheumatology (Oxford) 2001;40:652-55.
[Google Scholar]
|
| 30. |
Ermakova NA, Alekberova ZS. Retinal vasculitis in Behcet's disease. Vestn Oftalmol 2001;117:44-6.
[Google Scholar]
|
| 31. |
Saylan T, Mat C, Fresko I, Melikoðlu M. Behcet's disease in the Middle East. Clin Dermatol 1999;17:209-23.
[Google Scholar]
|
| 32. |
Dilsen N, Konice M, Aral O, Ocal L, Inanç M, Gül A. Comparative study of the skin pathergy test with blunt and sharp needles in Behcet's disease: Confirmed specificity but decreased sensitivity with sharp needles. Ann Rheum Dis 1993;52:823-5.
[Google Scholar]
|
| 33. |
Gul A, Esin S, Dilsen N, Koniçe M, Wigzell H, Biberfeld P. Immunohistology of skin pathergy reaction in Behcet's disease. Br J Dermatol 1995;132:901-7.
[Google Scholar]
|
| 34. |
Yazici h, Chamberlain MA, Tuzun Y, Yurdakul S,Müftüoglu A.Comparative study of the pathergy reaction among Turkish and British patients with Behcet's disease. Ann Rheum Dis 1984;43:74-5.
[Google Scholar]
|
| 35. |
Davatchi F, Chams-Davatchi C, Ghodsi Z, Shahram F, Nadji A, Shams H, et al. Diagnostic value of pathergy test in Behcet's disease according to the change of incidence over the time. Clin Rheumatol 2011;30:1151-5.
[Google Scholar]
|
| 36. |
Menashi S, Tribout B, Dosquet C, Le Toumelin P, Piette JC, Wechsler B, et al. Strong association between plasma thrombomodulin and pathergy test in Behcet disease. Ann Rheum Dis 2008;67:892-3.
[Google Scholar]
|
| 37. |
Davatchi F, Sadeghi Abdollahi B, Tehrani Banihashemi A, Shahram F, Nadji A, Shams H, et al. Colchicine versus placebo in Behçet's disease: Randomized, double-blind, controlled crossover trial. Modern Rheumatol 2009;19:542-9.
[Google Scholar]
|
| 38. |
Sharquie KE, Najim RA, Abu-Raghif AR. Dapsone in Behçet's disease: A double-blind, placebo-controlled, cross-over study.J Dermatol 2002;29:267-79.
[Google Scholar]
|
Fulltext Views
8,118
PDF downloads
2,271





