Translate this page into:
Beta-blockers in dermatology
2 Department of ENT and Head and Neck Surgery, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India
Corresponding Author:
Neel Prabha
Department of Dermatology, Venereology and Leprology, All India Institute of Medical Sciences, GE Road, Tatibandh, Raipur, Chhattisgarh
India
ripuneel@gmail.com
| How to cite this article: Prabha N, Chhabra N, Arora R. Beta-blockers in dermatology. Indian J Dermatol Venereol Leprol 2017;83:399-407 |
Introduction
Beta-blockers are drugs that block norepinephrine and epinephrine (adrenaline) from binding to beta-adrenergic receptors. They were first developed by Sir James Black in the United Kingdom in 1962 for which he was awarded the Nobel prize in 1988. Beta-blockers have been tried in a number of dermatological disorders. This review discusses their role in dermatology.
Classification
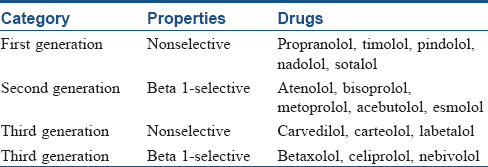
Beta-blockers are classified as nonsubtype-selective (” first generation”), beta 1-selective (”second generation”) and nonsubtype-selective or subtype-selective with additional cardiovascular actions (”third generation”) [Table - 1].[1] Propranolol is a pure antagonist and serves as a prototype agent.
Mechanism of Action
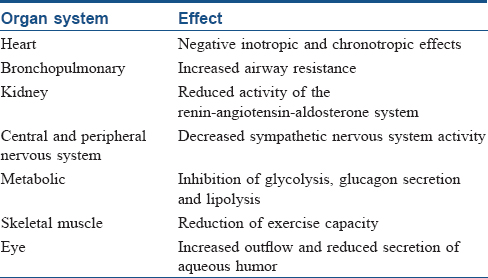
Beta-blockers antagonize the effects of sympathetic nerve stimulation or circulating catecholamines at beta-adrenoceptors which are widely distributed throughout body systems. There are three types of beta receptors. Beta-1 receptors are located in the heart, eyes and kidneys; beta-2 receptors are found in the lungs, gastrointestinal tract, liver, uterus, blood vessels and skeletal muscle and beta-3 receptors are located in fat cells. Beta-blockers differ in the type of beta receptors they block and therefore in their effects. Nonselective beta-blockers block beta 1 and 2 receptors and therefore affect the heart, blood vessels and air passages. Selective beta-blockers primarily block beta-1 receptors and therefore mostly affect the heart and not the air passages. Some beta-blockers, for example, pindolol have intrinsic sympathomimetic activity and can cause increases in blood pressure and heart rate. Labetalol and carvedilol block beta and alpha-1 receptors; blocking alpha receptors adds to their blood vessel-dilating effect. Effects of beta-blockers on different organs are described in [Table - 2].
Pharmacokinetics
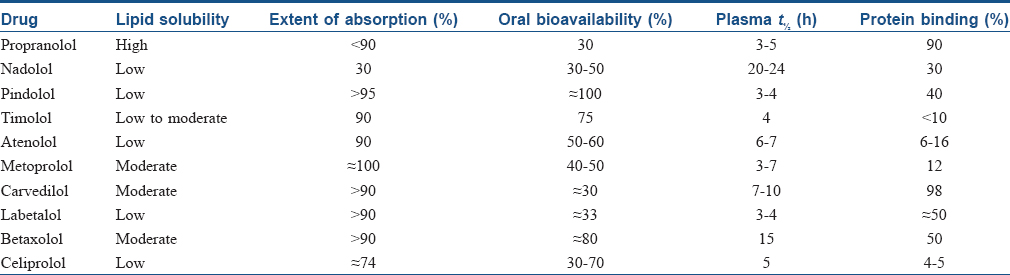
Beta-blockers vary in their degree of elimination by the kidney or the liver, usually with extensive first-pass metabolism. Lipid-soluble beta-blockers (labetalol, metoprolol, pindolol and propranolol) typically depend on hepatic metabolism for clearance, whereas water-soluble beta-blockers (atenolol) are cleared by the kidney. Drugs eliminated by the liver tend to exhibit wide interindividual variability in bioavailability. The half-lives of most beta-blockers are relatively short; those eliminated by the kidney tend to have longer half-lives. Pharmacokinetic properties of beta-blockers are listed in [Table - 3].[1]
Indications in Dermatology
Propranolol is US Food and Drug Administration approved for infantile hemangiomas [2] and other indications of beta-blockers are off label in dermatology.
Infantile hemangiomas
Infantile hemangiomas are benign vascular tumors. Use of beta-blockers for infantile hemangioma was first reported in 2008 when two infants taking propranolol for cardiac reasons had dramatic involution of their severe hemangiomas.[3] Since then, many studies have been published demonstrating the efficacy and safety of propranolol for infantile hemangiomas. The US Food and Drug Administration has approved oral propranolol for use in severe infantile hemangiomas.[2]
Ideally, propranolol should be initiated as early as possible, before rapid proliferation of the tumor. In one study, oral propranolol was given to 32 children at a mean age of 4.2 months.[4] Marqueling et al. in their meta-analysis of 1264 children found that oral propranolol was initiated at a mean age of 6.6 months (3 days to 10 years).[5] In a prospective study of 174 children, propranolol was administered at a mean age of 4.8 months.[6] However, there are several reports of it being effective even when initiated at the end of the growth phase or later.[7],[8] In a retrospective study of 42 children (above 1 year of age), oral propranolol diminished the size of those infantile hemangiomas that were well beyond the proliferative phase.[8] However, Holmes et al. did not observe any improvement in children with infantile hemangiomas in whom propranolol was started after 9 months of age.[9]
Therapeutic efficacy is observed immediately with propranolol therapy. Sans et al. used propranolol at a dose of 2–3 mg/kg/day for a mean total duration of 6.1 months in 32 children with severe infantile hemangiomas and noticed changes in color within 24 h, and softening in all the cases.[4] In ulcerated infantile hemangiomas, complete healing occurred in less than 2 months. Objective clinical and ultrasound evidence of long-term regression was seen in 2 months. Hermans et al. also reported improvement of respiratory symptoms in airway hemangioma within hours of treatment initiation with propranolol.[6]
In one randomized controlled trial, forty children between the ages of 9 weeks and 5 years with infantile hemangiomas were randomly assigned to receive oral propranolol (2 mg/kg/day divided into three doses daily) or placebo for 6 months.[10] In the propranolol group, infants younger than 6 months and children up to 5 years of age showed reduced volume, elevation and improved coloration in their localized and segmental infantile hemangiomas. Hemangioma growth stopped by week 4 in the propranolol group. Significant differences in the percent change in volume were seen between the groups, with the largest difference at week 12. A significant decrease in lesional redness and elevation occurred in the propranolol group at weeks 12 and 24, respectively.
In a retrospective chart review, propranolol was found to be more clinically effective than oral corticosteroids with better tolerance, minimal adverse effects and also resulted in fewer surgical interventions.[11] In this study, 110 patients were treated with either propranolol or corticosteroid for mean durations of 7.9 months and 5.2 months respectively. Fifty-six (82%) of 68 patients in the propranolol group achieved clearance of 75% or more, compared to 12 (29%) of 42 patients in the oral corticosteroid group (P < 0.01). Relapse after discontinuation of propranolol occurred in 2 of the 68 patients, but both again responded to re-initiation of propranolol. Surgical referrals after treatment were required in 8 (12%) patients in the propranolol group and 12 (29%) in the oral corticosteroid group (P < 0.01). In another study, 12 infantile hemangioma patients treated with propranolol were retrospectively matched with patients treated with oral prednisone according to type, location and size of the infantile hemangioma and age at the start of treatment.[12] At 1 month, there was moderate to good clinical improvement in all patients in the propranolol group. In the prednisone group, only one patient had moderate improvement while others showed slight (7/12) or no improvement or stabilization (3/12) from baseline, and one case worsened. At 6 months, the propranolol group showed good to excellent response in all cases, whereas 9 patients in the prednisone group showed slight to moderate response. A prospective study of 30 patients aged 1 week to 8 months treated with propranolol alone, prednisolone alone or propranolol with prednisolone concluded that propranolol produced a more consistent and rapid therapeutic response than oral prednisolone.[13] The effects of propranolol and prednisolone combined were comparable to but not more than propranolol alone, Prednisolone was associated with a higher number of complications.
Propranolol is an effective and well-tolerated treatment for ulcerated infantile hemangiomas as well. In a study of 20 children with ulcerated infantile hemangiomas, oral propranolol significantly decreased the duration of ulceration compared with the control group (8.7 vs. 22.4 weeks, P< 0.01).[14] In a retrospective study, the average time to complete healing of ulceration was 4.3 weeks in 30 of 33 infants with ulcerated infantile hemangiomas treated with propranolol, and the mean time to complete pain control was 14.5 days.[15]
Treatment duration with propranolol depends on the morphological type of hemangioma and the extent of involvement. Infantile hemangiomas need to be treated for a minimum period of 6 months. In deep and mixed infantile hemangiomas, the duration of treatment is longer because the proliferation phase lasts longer; therefore, treatment is continued until 12–16 months of age. In patients with ulceration, treatment is continued up to 9 to 12 months of age due to the risk of recurrence of ulceration.[16] A retrospective cohort study found that 12 months of treatment of infantile hemangiomas with oral propranolol was associated with a significantly lower rate of relapse than with shorter treatment (8 months or less).[17]
Upon discontinuation of propranolol, several reports have noted rebound growth or recurrence of the treated infantile hemangiomas. The systematic review by Marqueling et al. observed rebound growth in 17% of patients.[5] Another study reported rebound growth in 5 (19%) of 26 patients after discontinuation of propranolol, with the time from withdrawal to recurrence ranging from 0 to 6 months.[18] In the majority of cases, recurrence occurred in the deep component of the infantile hemangioma. Rebound growth has been attributed to early treatment withdrawal or a long proliferative phase of the infantile hemangioma. Re-initiation of propranolol is the treatment of choice for rebound growth.[19]
Some propranolol-resistant cases also have been reported.[10],[20],[21],[22] In a retrospective study, propranolol resistance (defined as continued growth during the proliferative phase or no decrease in the hemangioma during the postproliferative phase) after 4 weeks of oral propranolol was reported in 10 of 1130 cases.[22]
Consensus guidelines for initiation and monitoring of propranolol have been published.[23] For infants younger than 2 months of age, brief inpatient hospitalization for monitoring during induction of treatment is generally recommended. For infants over 2 months of age, propranolol can be initiated in an outpatient setting unless there are medical comorbidities or inadequate social support. A medical team with expertise in both the management of infantile hemangioma and the use of oral propranolol in infants provides the most optimal care to patients in need of systemic therapy with propranolol. The examination should be performed by a care provider with experience in evaluating infants and children. After a careful history and physical examination to exclude any reactive airway or cardiac disease, baseline heart rate and blood pressure are obtained. The consensus group recommends a target dose of 1 to 3 mg/kg per day, divided into 3 times daily dosing with a minimum of 6 hours between doses. Heart rate and blood pressure are monitored before the initial dose, 1 and 2 h following the same and after significant dose increase (>0.5 mg/kg/day), including at least 1 set of measurements after the target dose has been achieved. Parents should be informed of the risks of hypoglycemia and instructed to ensure that their child is fed regularly and to avoid prolonged fasts.
Other beta-blockers have also been found to be effective in infantile hemangiomas. In a retrospective cohort study, 44 patients were treated with nadolol for infantile hemangiomas. At least 50% improvement was noted in 42 (95%) patients and 75% improvement in 39 (89%) patients. The mean time to 50% and 75% improvement was 2.9 and 3.7 months, respectively.[24] In another study, switching to oral nadolol resolved the propranolol-related sleep disturbances in 5 of 7 patients without compromising efficacy.[25] In a randomized controlled study, atenolol was found to be as effective as propranolol.[26]
For superficial or small infantile hemangiomas, in which systemic therapy may not be indicated, topical beta-blockers, specifically timolol, have proven to be a useful alternative.[27],[28],[29],[30],[31],[32],[33],[34] In a multicentric retrospective study, timolol 0.5%–0.1% gel-forming solution was applied twice daily on superficial infantile hemangiomas; 72 of 73 patients exhibited some improvement, the mean duration of therapy was 3.4 months and treatment was well tolerated.[27] Another randomized controlled study of 41 infants with infantile hemangiomas found topical timolol maleate 0.5% gel to be safe and more effective than placebo.[28]
In yet another study, a dose of 0.25 mg timolol in gel form (manufactured from an ophthalmic formulation of timolol 0.5% eye drops) was used safely and to good effect in 9 children, including 6 preterm infants.[33] Topical timolol appeared to be more effective for plaques than for nodular lesions and for proliferating rather than involuting lesions.[34] However, a cautious approach is advisable with topical timolol since timolol is more potent than propranolol, and topical absorption would bypass first-pass metabolism in the liver.[35]
Topical propranolol (1%) ointment has also been found efficacious in superficial hemangiomas of the skin.[36] Forty-five children with 65 hemangiomas were treated with twice-daily application of 1% propranolol in a hydrophilic ointment. Treatment in the proliferative phase within the first 6 months of life induced regression in 59% and cessation of growth in 26% of the hemangiomas. No response or proliferation of subcutaneous components was observed in 15% of the hemangiomas. The treatment was well tolerated without side effects even in preterm infants and in children with numerous or large lesions. Bonifazi et al. reported treatment of 6 cases with topical 1% propranolol oil-based cream.[37] A retrospective chart review of 25 children with 28 lesions has been reported.[38] Topical 1% propranolol ointment was applied thrice daily for a mean duration of 21 weeks (range, 5–59 weeks). Of the 28 hemangiomas, 16 (57%) demonstrated good response, 9 (33%) showed a partial response and 3 (10%) had no response, with no systemic complication in any of the patients.
Intralesional propranolol, however, was found ineffective in shrinking infantile hemangiomas.[39] Six infants with small, noncomplicated infantile hemangiomas in areas of cosmetic concern were treated with 1 mg/mL propranolol solution at a dose of 0.2 ml/cm [2]. All hemangiomas stopped growing during therapy, but no significant changes in size or color were observed, even after repeated injections. No adverse events occurred. One patient whose hemangioma stopped growing during treatment presented with rebound growth after therapy cessation. The reason for this therapeutic failure was possibly because the vehicle was not appropriate for intralesional injection, leading to erratic absorption and no effect of local deposit and another theory was that the dose and number of injections were too low. Another possible explanation was that the patient's mean age was 7.3 months, as 80% of infantile hemangioma have reached their final size before 5 months of age, patients included could be considered “old” for this treatment.
The mechanism of action of beta-blockers in infantile hemangiomas has not been completely elucidated although they are believed to inhibit tumor growth by at least three distinct mechanisms: (1) vasoconstriction; (2) inhibition of angiogenesis or vasculogenesis by the downregulation of angiogenic factors, vascular endothelial growth factor and basic fibroblast growth factor and (3) induction of apoptosis of capillary endothelial cells.[40]
Other vascular tumors
Propranolol has also been tried in other vascular tumors with variable response. Hermans et al. in a case report described the effectiveness of propranolol in the treatment of a 6-week-old male with kaposiform hemangioendothelioma with Kasabach–Merritt syndrome.[41] The infant was treated with propranolol 2 mg/kg/day and vincristine 1.0 mg/m [2]/dose for 4 weeks. There was a dramatic clinical improvement and oral propranolol was continued alone for an additional 13 months. This report would suggest that propranolol is a potential treatment option for kaposiform hemangioendothelioma. However, in a later report by Chiu et al. of a case series of 11 patients of kaposiform hemangioendothelioma and tufted angioma with and without Kasabach–Merritt phenomenon, propranolol was found ineffective in nearly two-thirds of patients.[42] Only four patients responded and improvement was slow in three of these four patients. One patient partially improved on lower doses of propranolol but had a dramatic response to 3 mg/kg/day, suggesting that higher doses may be necessary. Arunachalam et al. successfully treated three patients of kaposiform hemangioendothelioma and one patient of congenital hemangiomas with Kasabach-Merritt syndrome with steroids, propranolol and vincristine in different combinations.[43] Choeyprasert et al. demonstrated successful treatment of mild pediatric Kasabach–Merritt phenomenon in a 5-week-old child with propranolol monotherapy resulting in both clinical and hematologic responses.[44]
Recently, Krakowski et al. reported a case in which a patient with tuberous sclerosis complex saw significant clinical improvement of her facial angiofibromas using a split-face comparison protocol of topical timolol 0.5% gel after full-field treatment with ablative fractional laser resurfacing and pulsed-dye laser.[45]
Pyogenic granulomas
Pyogenic granulomas or lobular capillary hemangiomas are common acquired vascular tumors. Although they are benign vascular proliferations, treatment is often sought because of recurrent episodes of bleeding and for cosmetic considerations. There are several treatment options including surgical removal, curettage and cauterization, laser and topical imiquimod; however, these treatments have been associated with pain, scarring and local side effects. Oral and topical beta-blockers have been found to be an effective and preferable alternative treatment to surgery for small pyogenic granulomas, particularly in children and young people. Khorsand et al. reported successful treatment of a 5-month-old child with a pyogenic granuloma on the cheek with topical timolol 0.5% gel for 24 weeks, without recurrence.[46] Wine et al. described similar results with topical timolol 0.5–2% applied 2–3 times daily for 12–24 weeks or systemic propranolol at 2 mg/kg twice daily for 6 months, or until resolution of the lesion.[47] Malik and Murphy successfully treated a teenager with a pyogenic granuloma on the finger with timolol 0.5% ophthalmic gel.[48] There were no reported adverse effects and the lesion completely resolved and had not recurred at 7 months. Recently, Gupta et al. studied the effect of topical timolol in ten patients with pyogenic granuloma, who received treatment with 0.5% timolol maleate ophthalmic solution applied four times a day, two drops per dose.[49] No other medication, topical or systemic, was given. Of the ten patients, four showed complete response within 3–24 days with no recurrence at 3-month follow-up. Three patients each showed partial or no response. No local or systemic side effects were reported in any of the patients.
Piraccini et al. verified the effectiveness of topical 1% propranolol cream on periungual and subungual pyogenic granuloma of hands and feet in ten patients.[50] Propranolol 1% cream was applied on the pyogenic granuloma lesions overnight under occlusion for a maximum of 45 days. It was found effective in frictional pyogenic granuloma and in drug-induced pyogenic granuloma of the fingers, while poor response was seen in drug-induced pyogenic granuloma of the toes and in pyogenic granuloma due to ingrown nail. Differences in response to therapy between fingernail and toenail lesions as well as between nail bed and lateral nail fold lesions were attributed to differences in expression of tissue markers and to the cream vehicle leading to inadequate drug penetration in toenail-fold, pyogenic granuloma where the skin is thicker than in the fingernails.
As pyogenic granulomas are benign vascular tumors, the mechanism of action of beta-blockers therein may be similar to that in infantile hemangiomas; vasoconstriction leads to inhibition of vascular growth factor and promotes cellular apoptosis.
Rosacea
Rosacea is a chronic disorder affecting the facial convexities, characterized by frequent flushing, persistent erythema and telangiectasia, interspersed by episodes of inflammation during which swelling, papules and pustules are evident. Flushing and burning are the most difficult features of rosacea to treat. Beta-blockers have been reported in isolated cases to be effective, but there are no evidence-based guidelines on their use in this condition.
Spoendlin et al. observed the association between the use of calcium channel blockers, beta-blockers and other antihypertensive drugs, and the incidence of rosacea.[51] Their results confirmed that calcium channel blockers increase the risk of rosacea, whereas beta-blocker use is associated with a slightly decreased risk of rosacea. Occasionally, an escalating dose of propranolol is helpful in reducing symptomatic flushing, but side effects often occur before its beneficial effect is evident.[52]
Craige and Cohen studied the use of propranolol in the control of flushing.[53] Though at a starting dose of 10 mg thrice a day, none of their 9 patients improved, 6 of them improved when doses were escalated to 20–30 mg thrice a day. At such high doses however, 3 patients withdrew from the study due to side effects.
Park et al. conducted a comparative study of propranolol, doxycycline and combination therapy in 78 patients with rosacea.[54] Twenty-eight patients were treated with propranolol, 22 with doxycycline and 28 with the combination of propranolol and doxycycline. The researchers found that in all groups there was an improvement from baseline in both patient global assessment and investigator global assessment. Combination therapy was most effective,(highest reduction in rosacea clinical score viz., 57.4% vs. 52.2% for doxycycline and 51.0% for propranolol), but the differences were not statistically significant. Mild and transient gastrointestinal disturbances were seen in three patients in the combination group, but the difference was not significant when compared to the other groups.
The effect of nadolol 40 mg daily versus placebo on both flushing provoked in a laboratory setting and spontaneous flushing was studied in 15 patients with erythematous telangiectatic rosacea.[55] No effect on objective measurements of provoked flushing was seen with nadolol, however there was an improvement in patient-reported spontaneous flushing.
Nadolol and propranolol can suppress flushing reactions, but the side effects of hypotension and bradycardia may pose problems. Carvedilol, a nonselective beta-adrenergic blocker with α1 blocking activity and potent antioxidant activity, has been found effective in low dose in treating refractory erythematous telangiectatic rosacea, with rapid symptom control and minimum side effects.[56],[57] Hsu et al. presented the results of carvedilol therapy in a case series.[57] Carvedilol (3.125–6.25 mg, 2–3 times a day) was used in 11 normotensive patients along with other medications (doxycycline, antihistamine and steroids) and the daily dose was titrated gradually up to 31.25 mg/day. All patients experienced a significant clinical improvement within 3 weeks (range: 3–21 days, mean: 10.5 days). Moreover, it also allowed other concurrent medications to be tapered or stopped. Side effects were minimal; only one patient had to discontinue treatment because of asymptomatic hypotension.
Beta-blockers may work in erythemato-telangiectatic rosacea by blocking beta-2 adrenergic receptors on the smooth muscle of cutaneous arterial blood vessels, resulting in vasoconstriction.
Angiolymphoid hyperplasia with eosinophilia
Angiolymphoid hyperplasia with eosinophilia is an uncommon, idiopathic disease that manifests as dermal or subcutaneous red or brown papules or nodules, most commonly on the head and neck. There may be accompanying serum eosinophilia and local lymphadenopathy. Treatment of angiolymphoid hyperplasia with eosinophilia is often challenging. A very promising treatment is oral propranolol. Horst et al. have reported a 32-year-old woman of angiolymphoid hyperplasia with eosinophilia who was started on oral propranolol (40 mg once daily).[58] Within 6 weeks, the patient noted improvement and within some months propranolol was stopped. One lesion recurred over a year later and propranolol was restarted; no new lesions occurred during 2 years of follow-up.
Topical timolol has also been shown to have some success in angiolymphoid hyperplasia with eosinophilia.[59] Authors believe that the success of propranolol is due to targeting the vascular proliferative element of angiolymphoid hyperplasia with eosinophilia.[58]
Malignant melanoma
Some observational studies have suggested a preventive and protective role for beta-blockers in melanoma.[60],[61],[62] Propranolol inhibits proliferation and induces apoptosis in primary cell cultures derived from both a primary and a metastasis of human melanoma, and in melanoma cell lines.[63] A study by De Giorgi et al. suggested that exposure to beta-blockers for 1 year or more was associated with a reduced risk of progression of thick malignant melanomas.[60] None of the thirty users of beta-blockers died over a median 2.5-year follow-up, whereas 24 out of 91 untreated patients died during the same period, and there was a 36% risk reduction for each year of beta-blocker use. In a Danish study with a median follow-up of 4.9 years and beta-blocker use within a median period of 90 days of melanoma diagnosis, both melanoma deaths and all-cause mortality were decreased.[61] Another study by De Giorgi et al. indicated that melanoma patients (79 patients) using beta-blockers, usually for hypertension, had improved overall survival after a median follow-up of 4 years.[62] For each year of beta-blocker use, the risk of death was reduced by 38%. However, a Dutch study found no significant effect of beta-blockers use, regardless of duration or dosage, on the survival of melanoma patients who started using beta-blockers after melanoma diagnosis.[64] Similarly in another population-based case–control study, beta-blocker use after malignant melanoma diagnosis was not found to be associated with reduced risk of death from melanoma.[65]
Adrenergic urticaria
Adrenergic urticaria is a rare but distinct type of physical urticaria characterized by wheals that are surrounded by white halos of vasoconstriction and by a positive response to intradermal adrenaline and noradrenaline injections.[66] Adrenergic urticaria has been treated successfully with variable doses of propranolol that can be increased up to 40 mg three times daily.[66],[67],[68],[69] The response to propranolol can be used to confirm the diagnosis as well as to prevent attacks. However, its exact mechanism of action in adrenergic urticaria is still unknown. The blockade of the beta-2 receptor on the mast cells might be implicated.[70] Alternatively, a direct central nervous system effect of propranolol which is known to cross the blood–brain barrier remains a possibility.[71]
Aquagenic pruritus
Aquagenic pruritus occurs during or after contact with water, involving intense itching without visible skin changes. Idiopathic aquagenic pruritus occurs without an underlying pathology (polycythemia vera, Hodgkin disease and blood disorders). The pathophysiology of idiopathic aquagenic pruritus is unknown, but pharmacological studies have shown that it is associated with local release of acetyl choline in the skin, mast-cell degranulation and raised blood histamine concentrations.[72] Increased cutaneous fibrinolytic activity both before and after contact with water, and inappropriate activation of the autonomic nervous system could be involved.[73],[74]
Treatment of this condition is nonspecific and highly unsatisfactory. Oral propranolol, 10 mg given 20–30 min before a bath, was found effective in reducing pruritus considerably in several cases of idiopathic aquagenic pruritus.[75] Nosbaum et al. in a study evaluated the efficacy and tolerability of propranolol in six patients with idiopathic aquagenic pruritus.[74] The patients received 10–40 mg/day of propranolol for 3 months, depending on their tolerance. In <7 days, complete remission was obtained in 4 patients and symptoms decreased by 90% in one patient. No relapse occurred during the 3 months of treatment, but after discontinuation of propranolol, clinical signs recurred in 5 patients. A cough was the only reported side effect induced by voluntarily doubling the dose from 20 to 40 mg/day. The patient who did not respond to propranolol had also been unresponsive to clonidine. On relapse, one patient was retreated with propranolol and experienced the same improvement seen with the first course.
Atenolol may be a preferred therapeutic option compared with propanolol, in view of its convenient once-a-day dosing and better side effect profile. Cao et al. reported a 36-year-old Indian female with aquagenic pruritus.[76] She was initially treated with propranolol with good response, and was subsequently switched to atenolol for convenient once-a-day dosing. Symptoms were well controlled for more than a year with no side effect experienced. Cao et al. propose that upon contact of the skin with water, as yet unknown mediator/s released stimulate dysfunctional and hyper-innervated C-nerve fibers which may result from a sodium channel defect, and that atenolol may exert its effect in aquagenic pruritus through blockage of these over-activated neuronal sodium channels.
Chronic ulcers
Beta-blockers have shown promising results in wound healing. Beta-2 receptors are located on keratinocytes and their blocking can improve keratinocyte migration, particularly to the wound center.[77] Beta-blockade also appears to enhance angiogenesis in the wound.[78] In a double-blinded, randomized controlled trial, patients who received oral propranolol had a shorter time to healing of superficial wounds, were quicker to receive skin grafts in deeper injuries and had shorter hospital stays, compared to the placebo group.[79] In a case series, five patients with chronic and recalcitrant wounds were treated with timolol 0.5% ophthalmic drops on either a daily or weekly basis for 4–8 weeks, dependent on the frequency of dressing changes for each specific wound, along with the standard of care. The medication was instilled with 1 drop for every 2 cm along the wound edge and allowed to dry for 2 min before dressing. All patients improved, including three who had complete healing.[80] Similar results are described in other case reports.[81],[82]
Pyoderma gangrenosum
Pyoderma gangrenosum is a rare, noninfectious neutrophilic dermatosis commonly associated with underlying systemic disease. There are currently no specific or uniformly effective therapies for pyoderma gangrenosum. Reepithelialization in pyoderma gangrenosum can be enhanced by enhancing keratinocyte migration with the help of beta-blockers.[77] Topical timolol was found effective in a patient with recalcitrant pyoderma gangrenosum.[83] Collagenase ointment and timolol 0.5% gel were applied daily under occlusion to the ulcer base and border, respectively, and after 2 months of treatment, the ulcer was completely reepithelialized.
Erythromelalgia
Erythromelalgia is a rare syndrome characterized by episodic burning pain, erythema and elevated temperature of the feet, hands or both. The syndrome can occur alone or with myeloproliferative or other diseases. Symptomatic responses have been reported with propranolol, usually at 10 mg 3 times a day. Higher dosages can be used. Propranolol's effect may lie in its ability to block beta-2 adrenergic vasodilator nerves in the skin. Except for timolol which has effects similar to propranolol, other beta-blocker drugs do not have this effect.[84],[85]
Hematohidrosis
Hematohidrosis is a rare idiopathic condition that manifests as self-limited episodes of spontaneous bloody discharge through intact skin or sweat gland orifices. Some theories have been proposed, including increased vascular pressure leading to the passage of blood cells through the ducts of the sweat glands, vasculitis of dermal vessels and exacerbated sympathetic activation leading to periglandular vessel constriction and subsequent expansion, allowing the passage of blood content into the ducts.[86] There is no specific management of this condition. Wang et al. used propranolol based on the hypothesis of sympathetic overactivity and found it effective.[87] They report a Chinese girl with frequent episodes of hematidrosis for more than 3 yearswhose bleeding problem was dramatically resolved by treatment with propranolol. She was given propranolol 10 mg twice daily and response was seen within 1 week. Treatment was continued for another month, the dose was reduced to 5 mg twice daily for another month and no relapse was seen in subsequent follow-up. There are other case reports of successful use of propranolol in this condition.[88],[89],[90]
Lichen planus
Despite beta-blockers having been incriminated in causing lesions of lichen planus, there is one published report about the usefulness of propranolol in patients of lichen planus. Pavithran accidentally noted remission of lichen planus when propranolol was started in a patient of lichen planus for associated hyperthyroidism.[91] This finding prompted him to study the effect of this drug in twenty patients with lichen planus. Cutaneous lesions responded very well to the treatment in 90% of the cases after 12 weeks, while mucous membrane lesions responded only partially and slowly. On the other hand, another twenty patients with lichen planus who received only oral pheniramine maleate showed persistence of skin lesions even after 12 weeks. Apart from one patient with an atopic background who developed bronchial asthma for the first time, there were no serious side effects noted with propranolol.
However, in contrast to the above study, propranolol was not at all found useful in another study by Kaur and Kumar.[92] Twenty-five patients of lichen planus were included in this study. Propranolol was given at a dose of 20 mg thrice daily orally to a maximum of 12 weeks. No improvement in cutaneous lesions or itch was observed in twenty patients at 4 weeks of therapy. Pruritus lessened in three patients after 3–4 weeks. In one patient, oral lesions healed completely, but cutaneous lesions flared up while on propranolol. Incidentally, there were three hypertensive patients being treated with propranolol for varying periods (1–3 years) who developed lichen planus while on propranolol.
Other indications in dermatology
There are isolated case-reports of beta-blockers used successfully in other dermatological diseases such as diffuse lymphangiomatosis,[93] granulomatous epulis [94] and papillary endothelial hyperplasia.[95]
Pretreatment Screening
Before starting propranolol, screening for risks associated with its use should be performed. History and a full clinical examination to rule out any contraindication to the use of a beta-blocker, a cardiac workup (ECG) and standard laboratory tests (complete blood counts, blood sugar, serum electrolytes and renal and liver function tests) are required.
Contraindications
Absolute contraindications to beta-blockers include uncompensated congestive heart failure, cardiogenic shock, severe sinus bradycardia, severe heart block, severe hyperactive airway disease, severe depression, active Raynaud's disease and hypersensitivity to beta-blockers. Relative contraindications are Prinzmetal's angina, mild asthma, peripheral vascular disease, diabetes mellitus (maintained on insulin and insulin releaser), thyrotoxicosis, untreated pheochromocytoma, heart failure, renal/hepatic impairment and myasthenia gravis.
Adverse Effects
Acebutolol, pindolol and sotalol are pregnancy category B medications. Propranolol, betaxolol, bisoprolol, carteolol, carvedilol, esmolol, metoprolol, nadolol and timolol are pregnancy category C medications. Only one beta-blocker, atenolol, is a pregnancy category D medication.
Adverse cutaneous drug reaction to beta-blockers
Although beta-blockers have been found effective in some dermatoses, there are certain adverse cutaneous drug reactions associated with their use as well. Beta-blockers are considered a major factor in triggering or aggravating psoriasis.[96],[97],[98],[99],[100] A retrospective study of patients with psoriasis found that of 26 patients treated with beta-blockers, 72% experienced exacerbations.[96] A study of 110 patients hospitalized with plaque psoriasis in the USA found that patients taking beta-blockers were significantly more likely to experience an exacerbation of psoriasis than patients not taking beta-blockers, especially those aged 50 years or over.[100]
The mechanism for the exacerbation of psoriasis with beta-blocker use is thought to be related to a blockade in the activation of the messenger system of cyclic adenosine 3',5'-cyclic monophosphate. This blockade results in reduced intracellular concentrations of calcium. This reduction may, in turn, cause an accelerated proliferation of keratinocytes or polymorphonuclear leukocytes, both of which may play a role in inducing or exacerbating psoriasis.[101]
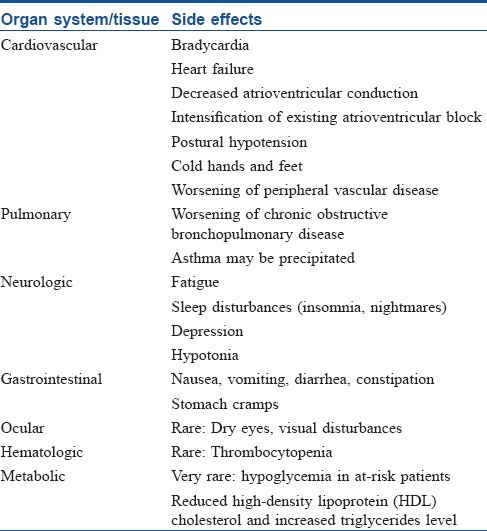
Other adverse cutaneous drug reactions and systemic side effects are summarized in [Table - 4][102],[103],[104] and [Table - 5], respectively.

Conclusion
The use of beta-blockers has revolutionized the treatment of infantile hemangiomas and has replaced systemic corticosteroids as first-line therapy for complicated hemangiomas of infancy. Beta-blockers can be used in other dermatological disorders as an adjuvant to established treatment modalities, or as monotherapy in resistant cases. However, more evidence from blinded randomized controlled trials and case–control studies are needed to determine their efficacy in other dermatoses.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. | Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, editor. The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. p. 277-333. [Google Scholar] |
| 2. | Food and Drug Administration. Orphan Drug Designation and Approvals. Silver Spring, MD: Food and Drug Administration; 2014. Available from: http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_Results_2.cfm?Index_Number=266708. [Last accessed on 2015 Sep 04]. [Google Scholar] |
| 3. | Lé auté -Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649-51. [Google Scholar] |
| 4. | Sans V, de la Roque ED, Berge J, Grenier N, Boralevi F, Mazereeuw-Hautier J, et al. Propranolol for severe infantile hemangiomas: Follow-up report. Pediatrics 2009;124:e423-31. [Google Scholar] |
| 5. | Marqueling AL, Oza V, Frieden IJ, Puttgen KB. Propranolol and infantile hemangiomas four years later: A systematic review. Pediatr Dermatol 2013;30:182-91. [Google Scholar] |
| 6. | Hermans DJ, Bauland CG, Zweegers J, van Beynum IM, van der Vleuten CJ. Propranolol in a case series of 174 patients with complicated infantile haemangioma: Indications, safety and future directions. Br J Dermatol 2013;168:837-43. [Google Scholar] |
| 7. | Schupp CJ, Kleber JB, Günther P, Holland-Cunz S. Propranolol therapy in 55 infants with infantile hemangioma: Dosage, duration, adverse effects, and outcome. Pediatr Dermatol 2011;28:640-4. [Google Scholar] |
| 8. | Zvulunov A, McCuaig C, Frieden IJ, Mancini AJ, Puttgen KB, Dohil M, et al. Oral propranolol therapy for infantile hemangiomas beyond the proliferation phase: A multicenter retrospective study. Pediatr Dermatol 2011;28:94-8. [Google Scholar] |
| 9. | Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg 2011;64:445-51. [Google Scholar] |
| 10. | Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics 2011;128:e259-66. [Google Scholar] |
| 11. | Price CJ, Lattouf C, Baum B, McLeod M, Schachner LA, Duarte AM, et al. Propranolol vs corticosteroids for infantile hemangiomas: A multicenter retrospective analysis. Arch Dermatol 2011;147:1371-6. [Google Scholar] |
| 12. | Bertrand J, McCuaig C, Dubois J, Hatami A, Ondrejchak S, Powell J. Propranolol versus prednisone in the treatment of infantile hemangiomas: A retrospective comparative study. Pediatr Dermatol 2011;28:649-54. [Google Scholar] |
| 13. | Malik MA, Menon P, Rao KL, Samujh R. Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: A randomized controlled study. J Pediatr Surg 2013;48:2453-9. [Google Scholar] |
| 14. | Hermans DJ, van Beynum IM, Schultze Kool LJ, van de Kerkhof PC, Wijnen MH, van der Vleuten CJ. Propranolol, a very promising treatment for ulceration in infantile hemangiomas: A study of 20 cases with matched historical controls. J Am Acad Dermatol 2011;64:833-8. [Google Scholar] |
| 15. | Saint-Jean M, Lé auté -Labrèze C, Mazereeuw-Hautier J, Bodak N, Hamel-Teillac D, Kupfer-Bessaguet I, et al. Propranolol for treatment of ulcerated infantile hemangiomas. J Am Acad Dermatol 2011;64:827-32. [Google Scholar] |
| 16. | Mansouri P, Hejazi S, Ranjbar M, Shakoei S. Propranolol in infantile hemangioma: A review article. J Skin Stem Cell 2014;1:e22884. [Google Scholar] |
| 17. | Giachetti A, Garcia-Monaco R, Sojo M, Scacchi MF, Cernadas C, Guerchicoff Lemcke M, et al. Long-term treatment with oral propranolol reduces relapses of infantile hemangiomas. Pediatr Dermatol 2014;31:14-20. [Google Scholar] |
| 18. | Bagazgoitia L, Hernández-Martín A, Torrelo A. Recurrence of infantile hemangiomas treated with propranolol. Pediatr Dermatol 2011;28:658-62. [Google Scholar] |
| 19. | Shehata N, Powell J, Dubois J, Hatami A, Rousseau E, Ondrejchak S, et al. Late rebound of infantile hemangioma after cessation of oral propranolol. Pediatr Dermatol 2013;30:587-91. [Google Scholar] |
| 20. | Buckmiller LM, Munson PD, Dyamenahalli U, Dai Y, Richter GT. Propranolol for infantile hemangiomas: Early experience at a tertiary vascular anomalies center. Laryngoscope 2010;120:676-81. [Google Scholar] |
| 21. | Goswamy J, Rothera MP, Bruce IA. Failure of propranolol in the treatment of childhood haemangiomas of the head and neck. J Laryngol Otol 2011;125:1164-72. [Google Scholar] |
| 22. | Caussé S, Aubert H, Saint-Jean M, Puzenat E, Bursztejn AC, Eschard C, et al. Propranolol-resistant infantile haemangiomas. Br J Dermatol 2013;169:125-9. [Google Scholar] |
| 23. | Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics 2013;131:128-40. [Google Scholar] |
| 24. | Randhawa HK, Sibbald C, Garcia Romero MT, Pope E. Oral nadolol for the treatment of infantile hemangiomas: A single-institution retrospective cohort study. Pediatr Dermatol 2015;32:690-5. [Google Scholar] |
| 25. | Bernabeu-Wittel J, Narváez-Moreno B, de la Torre-García JM, Fernández-Pineda I, Domínguez-Cruz JJ, Coserría-Sánchez F, et al. Oral nadolol for children with infantile hemangiomas and sleep disturbances with oral propranolol. Pediatr Dermatol 2015;32:853-7. [Google Scholar] |
| 26. | Ábarzúa-Araya A, Navarrete-Dechent CP, Heusser F, Retamal J, Zegpi-Trueba MS. Atenolol versus propranolol for the treatment of infantile hemangiomas: A randomized controlled study. J Am Acad Dermatol 2014;70:1045-9. [Google Scholar] |
| 27. | Chakkittakandiyil A, Phillips R, Frieden IJ, Siegfried E, Lara-Corrales I, Lam J, et al. Timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas: A retrospective, multicenter, cohort study. Pediatr Dermatol 2012;29:28-31. [Google Scholar] |
| 28. | Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics 2013;131:e1739-47. [Google Scholar] |
| 29. | Yu L, Li S, Su B, Liu Z, Fang J, Zhu L, et al. Treatment of superficial infantile hemangiomas with timolol: Evaluation of short-term efficacy and safety in infants. Exp Ther Med 2013;6:388-90. [Google Scholar] |
| 30. | Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: A pilot study. Arch Dermatol 2010;146:564-5. [Google Scholar] |
| 31. | Ni N, Langer P, Wagner R, Guo S. Topical timolol for periocular hemangioma: Report of further study. Arch Ophthalmol 2011;129:377-9. [Google Scholar] |
| 32. | Chambers CB, Katowitz WR, Katowitz JA, Binenbaum G. A controlled study of topical 0.25% timolol maleate gel for the treatment of cutaneous infantile capillary hemangiomas. Ophthal Plast Reconstr Surg 2012;28:103-6. [Google Scholar] |
| 33. | Moehrle M, Lé auté -Labrèze C, Schmidt V, Röcken M, Poets CF, Goelz R. Topical timolol for small hemangiomas of infancy. Pediatr Dermatol 2013;30:245-9. [Google Scholar] |
| 34. | Semkova K, Kazandjieva J. Topical timolol maleate for treatment of infantile haemangiomas: Preliminary results of a prospective study. Clin Exp Dermatol 2013;38:143-6. [Google Scholar] |
| 35. | McMahon P, Oza V, Frieden IJ. Topical timolol for infantile hemangiomas: Putting a note of caution in “cautiously optimistic”. Pediatr Dermatol 2012;29:127-30. [Google Scholar] |
| 36. | Kunzi-Rapp K. Topical propranolol therapy for infantile hemangiomas. Pediatr Dermatol 2012;29:154-9. [Google Scholar] |
| 37. | Bonifazi E, Colonna V, Mazzotta F, Balducci G, Laforgia N. Propranolol in rapidly growing hemangiomas. Eur J Pediatr Dermatol2008;18:185-92. [Google Scholar] |
| 38. | Xu G, Lv R, Zhao Z, Huo R. Topical propranolol for treatment of superficial infantile hemangiomas. J Am Acad Dermatol 2012;67:1210-3. [Google Scholar] |
| 39. | Torres-Pradilla M, Baselga E. Failure of intralesional propranolol in infantile hemangiomas. Pediatr Dermatol 2014;31:156-8. [Google Scholar] |
| 40. | Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br J Dermatol 2010;163:269-74. [Google Scholar] |
| 41. | Hermans DJ, van Beynum IM, van der Vijver RJ, Kool LJ, de Blaauw I, van der Vleuten CJ. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: A new indication for propranolol treatment. J Pediatr Hematol Oncol 2011;33:e171-3. [Google Scholar] |
| 42. | Chiu YE, Drolet BA, Blei F, Carcao M, Fangusaro J, Kelly ME, et al. Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr Blood Cancer 2012;59:934-8. [Google Scholar] |
| 43. | Arunachalam P, Kumar VR, Swathi D. Kasabach-Merritt syndrome with large cutaneous vascular tumors. J Indian Assoc Pediatr Surg 2012;17:33-6. [Google Scholar] |
| 44. | Choeyprasert W, Natesirinilkul R, Charoenkwan P. Successful treatment of mild pediatric Kasabach-Merritt phenomenon with propranolol monotherapy. Case Rep Hematol 2014;2014:364693. [Google Scholar] |
| 45. | Krakowski AC, Nguyen TA. Inhibition of angiofibromas in a tuberous sclerosis patient using topical timolol 0.5% gel. Pediatrics 2015;136:e709-13. [Google Scholar] |
| 46. | Khorsand K, Maier M, Brandling-Bennett HA. Pyogenic granuloma in a 5-month-old treated with topical timolol. Pediatr Dermatol 2015;32:150-1. [Google Scholar] |
| 47. | Wine Lee L, Goff KL, Lam JM, Low DW, Yan AC, Castelo-Soccio L. Treatment of pediatric pyogenic granulomas using ß-adrenergic receptor antagonists. Pediatr Dermatol 2014;31:203-7. [Google Scholar] |
| 48. | Malik M, Murphy R. A pyogenic granuloma treated with topical timolol. Br J Dermatol 2014;171:1537-8. [Google Scholar] |
| 49. | Gupta D, Singh N, Thappa DM. Is timolol an effective treatment for pyogenic granuloma? Int J Dermatol 2016;55:592-5. [Google Scholar] |
| 50. | Piraccini BM, Alessandrini A, Dika E, Starace M, Patrizi A, Neri I. Topical propranolol 1% cream for pyogenic granulomas of the nail: Open-label study in 10 patients. J Eur Acad Dermatol Venereol 2016;30:901-2. [Google Scholar] |
| 51. | Spoendlin J, Voegel JJ, Jick SS, Meier CR. Antihypertensive drugs and the risk of incident rosacea. Br J Dermatol 2014;171:130-6. [Google Scholar] |
| 52. | James WD, Berger TG, Elston DM. Acne. In: James WD, Berger TG, Elston DM, editors. Andrews' Diseases of the Skin: Clinical Dermatology, 11th ed. London: Saunders Elsevier; 2011. p. 228-246. [Google Scholar] |
| 53. | Craige H, Cohen JB. Symptomatic treatment of idiopathic and rosacea-associated cutaneous flushing with propranolol. J Am Acad Dermatol 2005;53:881-4. [Google Scholar] |
| 54. | Park JM, Mun JH, Song M, Kim HS, Kim BS, Kim MB, et al. Propranolol, doxycycline and combination therapy for the treatment of rosacea. J Dermatol 2015;42:64-9. [Google Scholar] |
| 55. | Wilkin JK. Effect of nadolol on flushing reactions in rosacea. J Am Acad Dermatol 1989;20(2 Pt 1):202-5. [Google Scholar] |
| 56. | Hsu CC, Lee JY. Carvedilol for the treatment of refractory facial flushing and persistent erythema of rosacea. Arch Dermatol 2011;147:1258-60. [Google Scholar] |
| 57. | Hsu CC, Lee JY. Pronounced facial flushing and persistent erythema of rosacea effectively treated by carvedilol, a nonselective ß-adrenergic blocker. J Am Acad Dermatol 2012;67:491-3. [Google Scholar] |
| 58. | Horst C, Kapur N. Propranolol: A novel treatment for angiolymphoid hyperplasia with eosinophilia. Clin Exp Dermatol 2014;39:810-2. [Google Scholar] |
| 59. | Chacon A, Mercer J. Successful management of angiolymphoid hyperplasia with eosinophilia in a split-face trial of topical tacrolimus and timolol solution. G Ital Dermatol Venereol 2016;151:436-40. [Google Scholar] |
| 60. | De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N, et al. Treatment with ß-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 2011;171:779-81. [Google Scholar] |
| 61. | Lemeshow S, Sørensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, et al. ß-Blockers and survival among Danish patients with malignant melanoma: A population-based cohort study. Cancer Epidemiol Biomarkers Prev 2011;20:2273-9. [Google Scholar] |
| 62. | De Giorgi V, Gandini S, Grazzini M, Benemei S, Marchionni N, Geppetti P. Effect of ß-blockers and other antihypertensive drugs on the risk of melanoma recurrence and death. Mayo Clin Proc 2013;88:1196-203. [Google Scholar] |
| 63. | Wrobel LJ, Le Gal FA. Inhibition of human melanoma growth by a non-cardioselective β-blocker. J Invest Dermatol 2015;135:525-31. [Google Scholar] |
| 64. | Livingstone E, Hollestein LM, van Herk-Sukel MP, van de Poll-Franse L, Nijsten T, Schadendorf D, et al. β-Blocker use and all-cause mortality of melanoma patients: Results from a population-based Dutch cohort study. Eur J Cancer 2013;49:3863-71. [Google Scholar] |
| 65. | McCourt C, Coleman HG, Murray LJ, Cantwell MM, Dolan O, Powe DG, et al. Beta-blocker usage after malignant melanoma diagnosis and survival: A population-based nested case-control study. Br J Dermatol 2014;170:930-8. [Google Scholar] |
| 66. | Shelley WB, Shelley ED. Adrenergic urticaria: A new form of stress-induced hives. Lancet 1985;2:1031-3. [Google Scholar] |
| 67. | Haustein UF. Adrenergic urticaria and adrenergic pruritus. Acta Derm Venereol 1990;70:82-4. [Google Scholar] |
| 68. | Maerens-Tchokokam B, Vigan M, Breuillard F, Vuitton DA, Girardin P, Laurent R. Guess what! Adrenergic urticaria. Eur J Dermatol 1999;9:137-8. [Google Scholar] |
| 69. | Vithayasai P, Vithayasai V. Adrenergic urticaria: A first report from Thailand. J Med Assoc Thai 1989;72:478-80. [Google Scholar] |
| 70. | Figueiredo A, Gonçalo M, Paiva I, Poiares-Baptista A. Adrenergic urticaria. Diabetes Care 1988;11:440-1. [Google Scholar] |
| 71. | Chedraoui A, Uthman I, Abbas O, Ghosn S. Adrenergic urticaria in a patient with anti-double-stranded DNA antibodies. Acta Derm Venereol 2008;88:263-6. [Google Scholar] |
| 72. | Greaves MW, Black AK, Eady RA, Coutts A. Aquagenic pruritus. Br Med J (Clin Res Ed) 1981;282:2008-10. [Google Scholar] |
| 73. | Lotti T, Steinman HK, Greaves MW, Fabbri P, Brunetti L, Panconesi E. Increased cutaneous fibrinolytic activity in aquagenic pruritus. Int J Dermatol 1986;25:508-10. [Google Scholar] |
| 74. | Nosbaum A, Pecquet C, Bayrou O, Amsler E, Nicolas JF, Bé rard F, et al. Treatment with propranolol of 6 patients with idiopathic aquagenic pruritus. J Allergy Clin Immunol 2011;128:1113. [Google Scholar] |
| 75. | Thomsen K. Aquagenic pruritus responds to propranolol. J Am Acad Dermatol 1990;22:697. [Google Scholar] |
| 76. | Cao T, Yong AA, Tan KB, Tey HL. Idiopathic aquagenic pruritus: Pathogenesis and effective treatment with atenolol. Dermatol Ther 2015;28:118-21. [Google Scholar] |
| 77. | Pullar CE, Rizzo A, Isseroff RR. Beta-Adrenergic receptor antagonists accelerate skin wound healing: Evidence for a catecholamine synthesis network in the epidermis. J Biol Chem 2006;281:21225-35. [Google Scholar] |
| 78. | Pullar CE, Le Provost GS, O'Leary AP, Evans SE, Baier BS, Isseroff RR. β2AR antagonists and β2AR gene deletion both promote skin wound repair processes. J Invest Dermatol 2012;132:2076-84. [Google Scholar] |
| 79. | Mohammadi AA, Bakhshaeekia A, Alibeigi P, Hasheminasab MJ, Tolide-ei HR, Tavakkolian AR, et al. Efficacy of propranolol in wound healing for hospitalized burn patients. J Burn Care Res 2009;30:1013-7. [Google Scholar] |
| 80. | Braun LR, Lamel SA, Richmond NA, Kirsner RS. Topical timolol for recalcitrant wounds. JAMA Dermatol 2013;149:1400-2. [Google Scholar] |
| 81. | Tang JC, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg 2012;38:135-8. [Google Scholar] |
| 82. | Lev-Tov H, Dahle S, Moss J, Isseroff RR. Successful treatment of a chronic venous leg ulcer using a topical beta-blocker. J Am Acad Dermatol 2013;69:e204-5. [Google Scholar] |
| 83. | Liu DY, Fischer R, Fraga G, Aires DJ. Collagenase ointment and topical timolol gel for treating idiopathic pyoderma gangrenosum. J Am Acad Dermatol 2014;71:e225-6. [Google Scholar] |
| 84. | Bada JL. Treatment of erythromelalgia with propranolol. Lancet 1977;2:412. [Google Scholar] |
| 85. | Serratrice G, Godde JL, Schiano A. Treatment of erythromelalgia with beta-blocking agents. Nouv Presse Med 1977;6:3973. [Google Scholar] |
| 86. | Manonukul J, Wisuthsarewong W, Chantorn R, Vongirad A, Omeapinyan P. Hematidrosis: A pathologic process or stigmata. A case report with comprehensive histopathologic and immunoperoxidase studies. Am J Dermatopathol 2008;30:135-9. [Google Scholar] |
| 87. | Wang Z, Yu Z, Su J, Cao L, Zhao X, Bai X, et al. A case of hematidrosis successfully treated with propranolol. Am J Clin Dermatol 2010;11:440-3. [Google Scholar] |
| 88. | Praveen BK, Vincent J. Hematidrosis and hemolacria: A case report. Indian J Pediatr 2012;79:109-11. [Google Scholar] |
| 89. | Bhattacharya S, Das MK, Sarkar S, De A. Hematidrosis. Indian Pediatr 2013;50:703-4. [Google Scholar] |
| 90. | Deshpande M, Indla V, Kumar V, Reddy IR Child who presented with hematohidrosis (sweating blood) with oppositional defiant disorder. Indian J Psychiatry 2014;56:289-91. [Google Scholar] |
| 91. | Pavithran K. Treatment of lichen planus with propranolol. Indian J Dermatol Venereol Leprol 1986;52:71-3. [Google Scholar] |
| 92. | Kaur I, Kumar B. Failure of propranolol in lichen planus. Indian J Dermatol Venereol Leprol 1991;57:53. [Google Scholar] |
| 93. | Ozeki M, Fukao T, Kondo N. Propranolol for intractable diffuse lymphangiomatosis. N Engl J Med 2011;364:1380-2. [Google Scholar] |
| 94. | Liu C, Qin ZP, Fan ZN, Zhao WJ, Wang YM, Wei FC, et al. New treatment strategy for granulomatous epulis: Intralesional injection of propranolol. Med Hypotheses 2012;78:327-9. [Google Scholar] |
| 95. | Prey S, Haberstroh G, Vergier B, Taïeb A, Wassef M, Ezzedine K, et al. Successful treatment of intravascular papillary endothelial hyperplasia (IPEH) by the beta-adrenergic antagonist nebivolol. Br J Dermatol 2012;166:1147-9. [Google Scholar] |
| 96. | Gold MH, Holy AK, Roenigk HH Jr. Beta-blocking drugs and psoriasis. A review of cutaneous side effects and retrospective analysis of their effects on psoriasis. J Am Acad Dermatol 1988;19(5 Pt 1):837-41. [Google Scholar] |
| 97. | Halevy S, Livni E. Psoriasis and psoriasiform eruptions associated with propranolol – The role of an immunological mechanism. Arch Dermatol Res 1991;283:472-3. [Google Scholar] |
| 98. | Steinkraus V, Steinfath M, Mensing H. Beta-adrenergic blocking drugs and psoriasis. J Am Acad Dermatol 1992;27(2 Pt 1):266-7. [Google Scholar] |
| 99. | Halevy S, Livni E. Beta-adrenergic blocking drugs and psoriasis: The role of an immunologic mechanism. J Am Acad Dermatol 1993;29:504-5. [Google Scholar] |
| 100. | Cohen AD, Bonneh DY, Reuveni H, Vardy DA, Naggan L, Halevy S. Drug exposure and psoriasis vulgaris: Case-control and case-crossover studies. Acta Derm Venereol 2005;85:299-303. [Google Scholar] |
| 101. | O'Brien M, Koo J. The mechanism of lithium and beta-blocking agents in inducing and exacerbating psoriasis. J Drugs Dermatol 2006;5:426-32. [Google Scholar] |
| 102. | Breathnach SM. Drug reactions. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford: Wiley-Blackwell; 2010. p. 75.1-75.177. [Google Scholar] |
| 103. | Greiner D, Schöfer H, Milbradt R. Reversible transverse overcurvature of the nails (pincer nails) after treatment with a beta-blocker. J Am Acad Dermatol 1998;39:486-7. [Google Scholar] |
| 104. | Bostanci S, Ekmekci P, Akyol A, Peksari Y, Gürgey E. Pincer nail deformity: Inherited and caused by a beta-blocker. Int J Dermatol 2004;43:316-8. [Google Scholar] |
Fulltext Views
20,585
PDF downloads
5,428





