Translate this page into:
Bilateral oral melanoacanthoma in an Indian boy
2 Department of Dermatology, Madurai Medical College, Madurai, India
Correspondence Address:
A S Krishnaram
256a Kamarajar Salai, Opp. to new Ananda Metals, Madurai - 625 009, Tamil Nadu
India
| How to cite this article: Geetha T, Rani G G, Krishnaram A S. Bilateral oral melanoacanthoma in an Indian boy. Indian J Dermatol Venereol Leprol 2011;77:210-212 |
Sir,
An 8-year-old boy was examined in a skin out-patient department for pigmented lesions in the oral cavity of 2 months duration. Lesions started as asymptomatic pigmented macules involving both the buccal mucosae with rapid increase in size in the first few weeks. There was no history of dental caries, trauma or any oral surgery. There was no history of systemic complaints or other skin diseases, and any similar history was not present in the family.
Examination revealed two patches, one on either side, involving the buccal, alveolar and gingival mucosae [Figure - 1]. Patches were brownish black, uniformly pigmented, each measuring 5 Χ 3 cm, with margins fading into the normal mucosa and were nonpalpable. Oral cavity was otherwise normal.
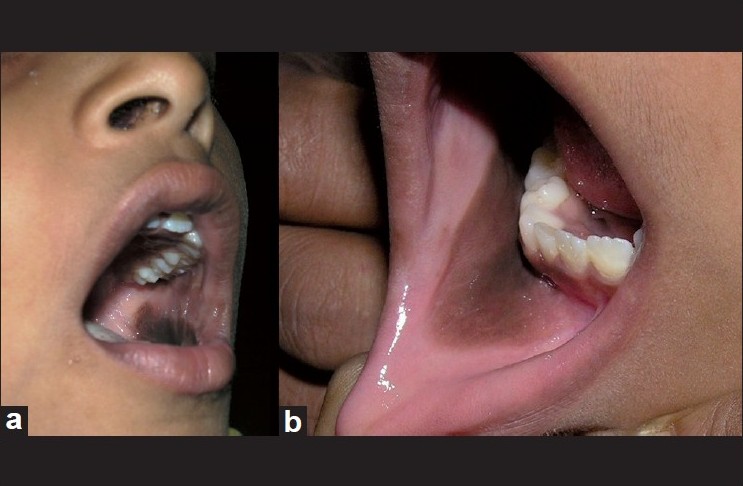 |
| Figure 1: (a) Brown black patch seen in left buccal mucosa. (b) Brown black patch fading with the normal mucosa in the right alveolar mucosa |
Basic investigations comprising complete hemogram, blood sugar, blood urea, serum creatinine, and ultrasonogram abdomen were normal. Due to the rapid growth, an incisional biopsy was done to rule out the possibility of melanoma.
Histopathological examination under hematoxylin and eosin showed proliferation and dispersion of dendritic melanocytes in variable numbers in the entire thickness of acanthotic epithelium [Figure - 2]. Melanophages and patchy chronic inflammatory cell collections were seen in the upper lamina propria [Figure - 2]. There was no cytologic atypia [Figure - 3]. The melanocytes were confirmed by Masson-Fontana silver stain [Figure - 4].
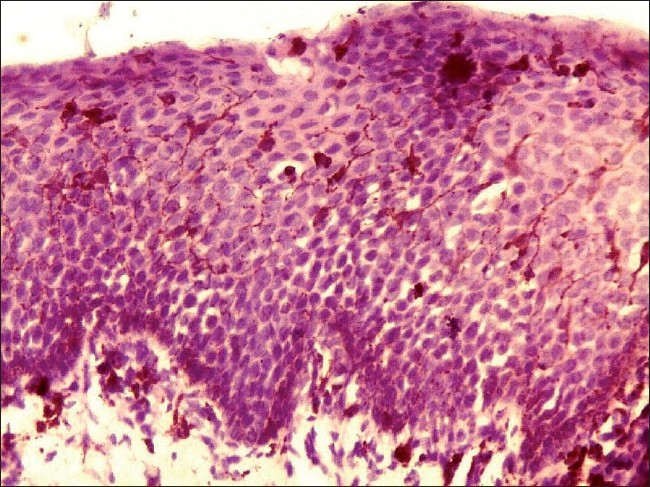 |
| Figure 2: Histopathology of oral melanoacanthoma. Hematoxylin and eosin section showing dispersion of pigment-laden dendritic melanocytes in acanthotic epithelium. Melanophages and scanty inflammatory cells are seen in the lamina propria (�100) |
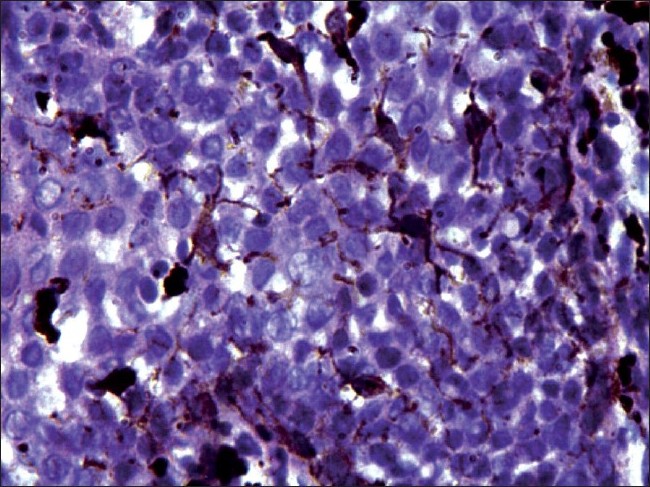 |
| Figure 3: Histopathology of oral melanoacanthoma. High power view showing pigment-laden dendritic melanocytes with no cytological atypia (×200) |
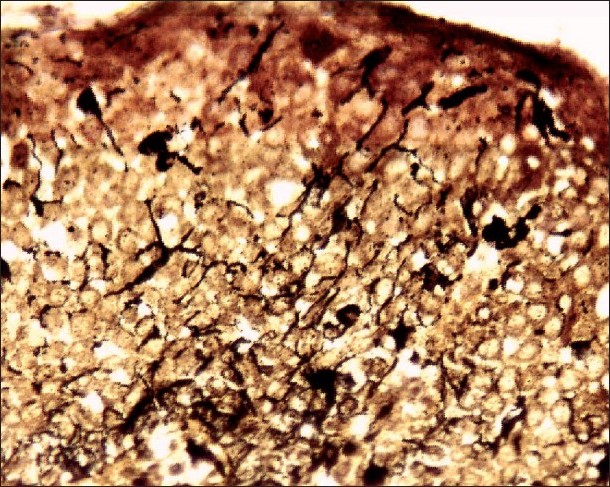 |
| Figure 4: Special staining with Masson-Fontana stain showing melanin laden dendritic melanocytes black amidst acanthotic epithelium (×200) |
A diagnosis of bilateral melanoacanthoma of the oral cavity (OMA) was made. Malignant melanoma (MM) and oral melanotic macules (OMM) were considered.
Since the first report of OMA by Matsuoka et al. in 1979, [1] at least 65 or more cases have been described till date. [2] It occurs mainly in Blacks, followed by Caucasians, Hispanics, and rarely, in Asians. It affects females more than males (3:1) within a wide range of age groups (5-77 years) with a mean of 33.7 years. [3] As far as could be ascertained, only one case from India and two cases of Asian Indians have been reported so far. [4] Besides being the youngest Indian to be reported, he is probably the third youngest in the history of the disease, the youngest being a 5-year-old girl. [3]
OMA is a benign pigmented disorder of the oral mucosa, characterized by simultaneous proliferation of both melanocytes and keratinocytes. It is considered to be of reactive origin. To emphasize the non-neoplastic nature of the disease, Tomich and Zunt suggested the term melanoacanthosis while reserving the designation "melanoacanthoma" for cutaneous tumors. [5]
Melanocytes are dendritic cells with small, dark staining nucleus and a clear cytoplasm. They constitute nearly 10% of the basal layer and are randomly dispersed within it. Melanocytes are normally seen only in the basal layer. The defining histology in OMA is proliferation and dispersion of benign melanocytes throughout the acanthotic epithelium. Spongiosis, melanophages, and submucosal chronic inflammatory infiltrate admixed with eosinophils are other findings.
OMA is clinically characterized by a solitary large dark brown to black patch involving, in order of frequency, the buccal mucosa, palate, lips and gingiva. OMA is usually a solitary lesion but multiple and bilateral lesions have been reported in 18.9% of cases. [2]
Multifocal OMA, as compared to the solitary type, has an equal gender distribution and involves the palate more frequently. [1] In this case, the palate was not involved. Gingival MA is a subset of OMA with mean age of 43.8 years with 80% affecting Black females. [2] The alarming rapid growth rate of OMA mimics melanoma clinically. It has a tendency to follow a traumatic event with spontaneous regression or resolution following biopsy. [2]
OMM closely resembles OMA, constituting 86% of solitary melanocytic lesions of the mouth compared to 0.9% of OMA. [6] Histologically, it differs by having only an in situ increased production of melanin by melanocytes, which are normal in number and distribution. Melanotic macules of labial mucosa, a variant of OMM, in addition, show melanocytic proliferation in the basal layer. [6]
The other pigmentary disorders of the oral cavity like melanocytic nevus, atypical melanocyticproliferation/hyperplasia and melanomas are differentiated by their distinct histopathology.
A diagnosis of OMA can be made solely on the basis of histological features and special staining, as done in this case. The immunohistochemical profile of these lesions is limited to the melanocytic markers, but is not necessary for diagnosis, as strong reactivity to HMB-45 and S100 is seen in both OMA and MM. [6]
Solitary or multifocal conservative biopsy is sufficient for identification and definitive treatment. Surgical intervention may be needed for symptomatic OMA. Recently, argon plasma coagulation has been tried successfully. [7] At 6 weeks follow-up, the biopsied lesion showed partial regression with no occurrence of new lesions.
This case is reported for its rare occurrence in Asians, for being that of the youngest Indian and Asian patient, and for being the second case reported from India. This case highlights the infrequent multifocal origin and bilateral presentation; the importance of biopsy to rule out MM is also emphasized. We also hope to add to the minimal existing literature on OMA.
| 1. |
Marocchio LS, Junior DS, de Sousa SC, Fabre RF, Raitz R. Multifocal diffuse oral melanoacanthoma: A case report. J Oral Sci 2009;51:463-6.
[Google Scholar]
|
| 2. |
Brooks JK, Sindler AJ, Papadimitriou JC, Francis LA, Scheper MA. Multifocal melanoacanthoma of the gingiva and hard palate. J Periodontol 2009;8:527-32.
[Google Scholar]
|
| 3. |
Fornatora ML, Reich RF, Haber S, Solomon F, Freedman PD. Oral melanoacanthoma: A report of 10 cases, review of the literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol 2003;25:12-5.
[Google Scholar]
|
| 4. |
Lakshminarayanan V, Ranganathan K. Oral melanoacanthoma: A case report and Review of literature. J Med Case Reports 2009;3:11.
[Google Scholar]
|
| 5. |
Tomich CE, Zunt SL. Melanoacanthosis (melanoacanthoma) of the oral mucosa. Dermatol Surg Oncol 1990;16:231-6.
[Google Scholar]
|
| 6. |
Carlos-Bregni R, Contreras E, Netto AC, Mosqueda-Taylor A, Vargas PA, Jorge J, et al. Oral melanoacanthoma and oral melanotic macule: A report of 8 cases, review of the literature, and immunohistochemical analysis. Med Oral Patol Oral Cir Bucal 2007;12:374-9.
[Google Scholar]
|
| 7. |
Andrews BT, Trask DK. Oral melanoacanthoma: A case report, a review of the literature, and a new treatment option. Ann Otol Rhinol Laryngol 2005;11:677-80.
[Google Scholar]
|
Fulltext Views
4,403
PDF downloads
1,724





