Translate this page into:
Bimekizumab: A novel FDA approved dual IL-17 A/F inhibitor for moderate to severe psoriasis
Corresponding author: Dr. Simin Muhammed Kutty, Department of Paediatric Dermatology, Aspire Children’s Speciality Centre, Erahnipalam, Calicut, Kerala, India. siminmk@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ashraf F, Kutty SM. Bimekizumab: A novel FDA approved dual IL-17 A/F inhibitor for moderate to severe psoriasis. Indian J Dermatol Venereol Leprol. 2025;91:226-30. doi: 10.25259/IJDVL_867_2024
Introduction
Psoriasis is a chronic inflammatory skin disease affecting millions globally, the estimated worldwide prevalence being 2–3%. In India, the prevalence of psoriasis ranges from 0.44% to 2.8%, affecting both rural and urban populations.1 Apart from physical discomfort, psoriasis significantly impairs the quality of life by inducing symptoms such as itching, pain, and psychological stress. Its chronic nature and the recurrent inflammatory cycles are associated with several systemic comorbidities such as psoriatic arthritis, cardiovascular disease, and metabolic syndrome. Recent advancements in understanding the immunology of psoriasis have prompted the development of newer biologic therapies, particularly monoclonal antibodies. These biologics target specific cytokines, such as interleukin-17 (IL-17) and IL-23, which are key drivers of the psoriatic inflammatory pathways.
Bimekizumab is a novel biologic targeting both IL-17A and IL-17F.2 Unlike conventional biologics that typically focus on IL-17A or other single inflammatory pathways, bimekizumab’s unique dual inhibition aims to comprehensively suppress the underlying inflammatory processes implicated in psoriasis and related conditions.
Structure
Bimekizumab is an advanced humanised dual monoclonal antibody, which is engineered using recombinant DNA (rDNA) technology in Chinese hamster ovary (CHO) cells. With a molecular weight of approximately 150 kDa, bimekizumab consists of two identical antibody units. This specific structure enables bimekizumab to effectively bind to and inhibit IL-17A and IL-17F, which are pivotal cytokines involved in the pathogenesis of psoriasis and psoriatic arthritis [Figure 1].2
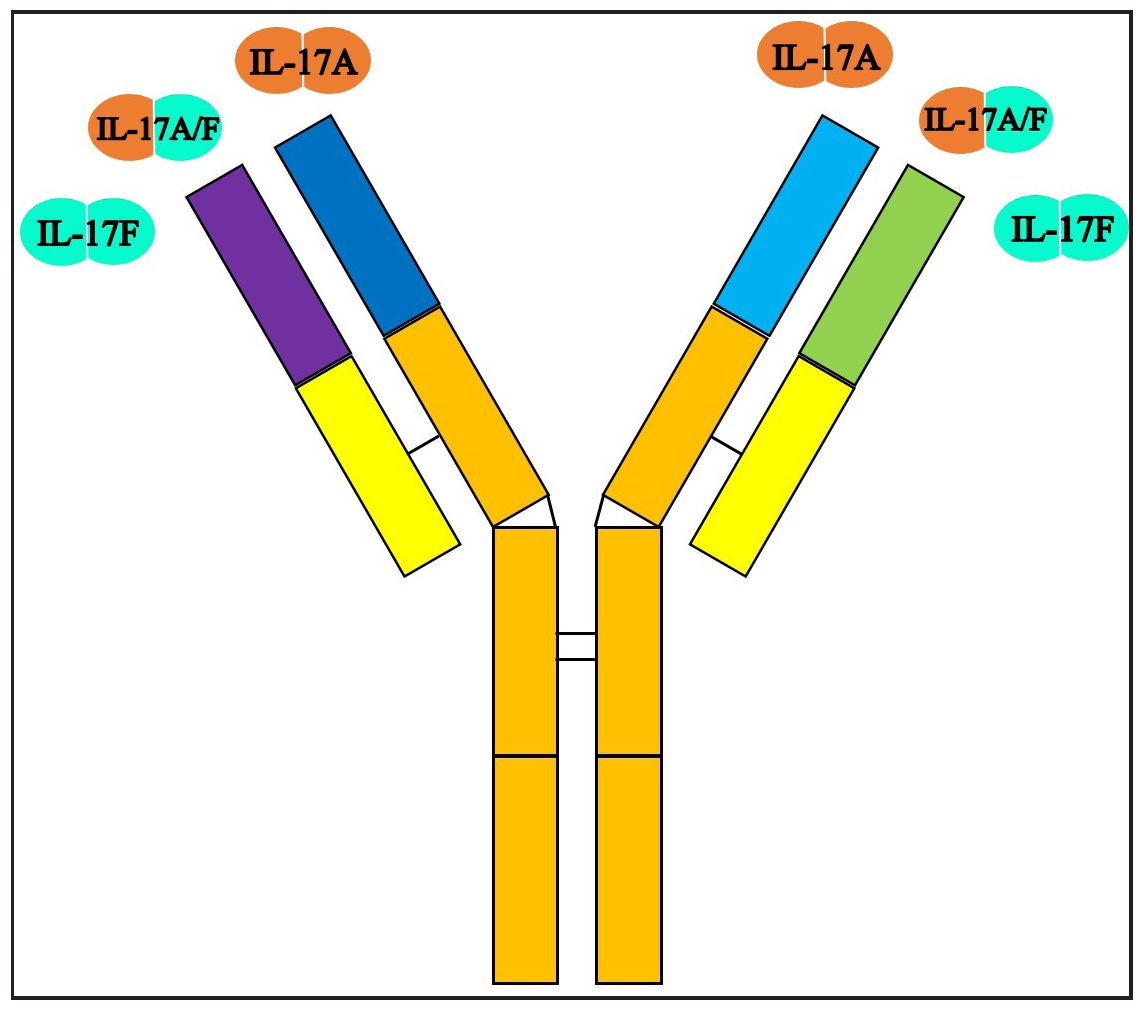
- Structure of bimekizumab. It can bind to IL-17F homodimers, IL -17A homodimers and IL-17 A/F heterodimers.
Mechanism of action
Bimekizumab is designed to inhibit both IL-17A and IL-17F cytokines, which are central to the IL-17/23 pathway involved in psoriasis. IL-17A, primarily produced by T helper 17(Th17) cells, binds to IL-17 receptors in the skin, triggering an inflammatory cascade that leads to psoriasis symptoms. Drugs like secukinumab and ixekizumab specifically target IL-17A, aiming to block its pro-inflammatory effects. In contrast, brodalumab targets IL17 A receptor, another crucial component of the IL-17 signaling pathway [Figure 2].2
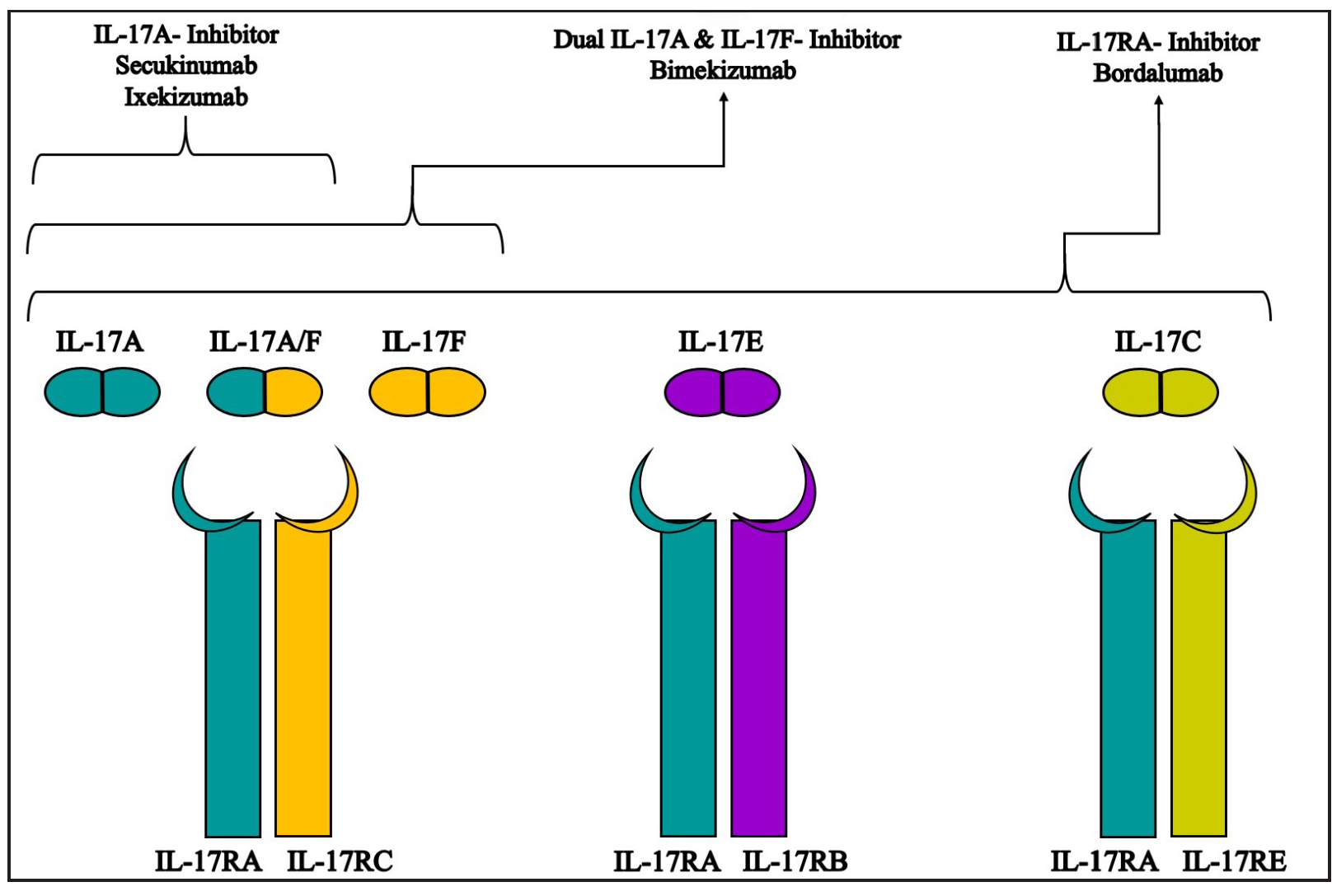
- Schematic representation of the mechanism of action of various monoclonal antibodies against IL 17 isoforms and receptors.
Although IL-17F is less biologically potent than IL-17A, it is found at higher levels in psoriatic plaques and serum.2 IL-17F also binds to IL-17A and IL-17C receptors, contributing to the inflammatory processes. By targeting both cytokines, bimekizumab offers a more comprehensive suppression of inflammation, potentially leading to faster and more sustained improvements. The fastest documented case of clearance in the literature involved a patient receiving bimekizumab, achieving Psoriasis Area and Severity Index (PASI) 90 within 72 hours and complete clearance (PASI 100) within a week, with itch resolution in just 24 hours.3
Indications
Bimekizumab is approved for moderate to severe plaque psoriasis in adults who require systemic therapy or phototherapy in the European Union (EU) 2021 and the United States Food and Drug Administration (USFDA) 2023. In September 2024, USFDA has approved it for psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis.4,5
It is used off-label for conditions like hidradenitis suppurativa, nail psoriasis, scalp psoriasis, genital psoriasis, erythrodermic psoriasis, pustular psoriasis, palmoplantar pustulosis and refractory pityriais rubra pilaris.6–12 Bimekizumab has shown efficacy in patients with complex medical conditions, including those on haemodialysis.13
Pharmacokinetics and drug interaction
Following subcutaneous injection, bimekizumab achieves a median peak plasma concentration of 25 μg/mL within three days. It has 70% bioavailability and a mean volume of distribution of 11.2L. The drug undergoes degradation through catabolic pathways into small peptides. Its primary elimination half-life averages 23 days.14 Bimekizumab can influence cytokine levels, impacting the synthesis of cytochrome P450 (CYP450) enzymes. Therefore, the dosage of CYP450 substrate drugs (e.g. warfarin, cyclosporine) needs appropriate adjustment.
Composition, storage, and cost
Bimekizumab, marketed under the tradename BimzelX®, is available as a clear to slightly opalescent solution ranging from colorless to pale yellow-brown. It should be stored at 2–8°C and can be kept at room temperature for up to 30 days.14 Each 160 mg/mL dose costs INR 57,953.56.
Dosage and administration
Before starting bimekizumab, patients should be assessed for infections, including active tuberculosis. Treatment should not be initiated if there is an active infection; until it is resolved or adequately treated. The risks and benefits should be weighed in patients with chronic or recurrent infections and they should be educated seek medical advice if infection symptoms arise. Close monitoring and therapy discontinuation is necessary if an infection develops or in case of treatment failure. Liver function must be monitored before beginning bimekizumab, checking alkaline phosphatase, bilirubin, and transaminase levels. If liver enzymes increase, therapy must be interrupted until the liver injury is ruled out. If transaminase and bilirubin levels remain elevated, bimekizumab should be permanently discontinued. It is contraindicated in patients with acute liver disease or cirrhosis.
Patients with inflammatory bowel disease (IBD) should not use bimekizumab if they have active disease. One should monitor for IBD symptoms during treatment and discontinue if new or worsening symptoms occur. Mental health should be monitored during therapy for emerging or worsening depression, suicidal thoughts, or mood changes. All age-appropriate vaccinations must be completed before starting bimekizumab. Live vaccines should be avoided during treatment.
Bimekizumab comes in 160 mg/1 mL vials, available as pre-filled syringes or auto-injectors. Two 160 mg subcutaneous injections need administrations per session, with injection sites at least two inches (5 cm) from the navel and psoriatic plaques, typically on the arms, thighs, or abdomen. Initial dosing is 320 mg at weeks 0, 4, 8, 12, and 16, followed by maintenance doses every eight weeks. For patients over 120 kg, maintenance may be every four weeks. Bimekizumab can be administered by healthcare professionals or trained patients. If a dose is missed, administer as soon as possible and resume the regular schedule.14
Special considerations
Pregnancy: Limited data on risks to mother and fetus. Animal studies in cynomolgus monkeys showed no maternal toxicity or foetal malformations. Risks and benefits must be weighed for live vaccines for infants exposed in utero.
Lactation: No data on bimekizumab in breast milk or its effects on breastfeeding infants.
Paediatric use: Safety and effectiveness in paediatric populations is not established.
Geriatric use: No identified differences in safety and effectiveness for those over 65 compared to younger adults.
Efficacy in clinical trials
Clinical trials like BE READY, BE VIVID, BE SURE, and BE RADIANT consistently demonstrated robust efficacy with high rates of PASI 90 and PASI 100 responses. These trials underscored bimekizumab’s potent therapeutic effects, highlighting its superiority over the existing treatments such as ustekinumab, adalimumab, and secukinumab. Bimekizumab not only achieved rapid and sustained clearance of skin symptoms but also significantly improved patient-reported outcomes.15–18 The data from these trials are outlined in Table 1. The BE BRIGHT trial, an open-label extension study designed to evaluate the maintenance of treatment response over three years in patients with moderate to severe plaque psoriasis, consistently showed that a significant proportion of patients who initially responded by week 16 maintained high levels of clinical response throughout the study period.19
| Study | Number of patients | Drug and dosage | Duration in weeks | Results in percentage |
|---|---|---|---|---|
|
BE READY (BKZ vs placebo) |
Group A: 349 Group B: 86 |
Group A:BKZ 320mg Q4W Group B: Placebo Q4W |
16 W |
4W,PASI 75 Group A: 76% Group B: 1% 16W, PASI 90 Group A: 91% Group B: 1% 16W, IGA score 0 or 1 Group A: 93% Group B: 1% |
|
Group A: 106 Group B:100 Group C: 105 |
Group A: BKZ 320mg Q4W Group B: BKZ 320mg Q8W Group C: Placebo Q4W |
16W TO 56 W |
16W, PASI 90 Group A, B: 89% Group C: 16% |
|
|
BE SURE (BKZ vs ADA) |
Group A: 158 Group B:161 Group C: 159 |
Group A: BKZ 320mg Q4W Group B: BKZ 320mg Q4W up to 16W, then Q8W Group C: ADA 40mg Q2W up to 24W, then BKZ 320 mg Q4W |
56W |
16w, PASI 90 Group A,B: 86.2% Group C: 47.2% 16 W, IGA score 0 or 1 Group A,B: 85.3% Group C: 57.2% |
|
BE VIVID (BKZ vs UST vs placebo) |
Group A: 83 Group B:321, 395 from 16W Group C: 163 |
Group A: Placebo Group B: BKZ 320mg Q4W Group C: UST 45/90 mg 0, 4 and QW12 |
52W |
16W, PASI 90 Group A: 5% Group B:85% Group C: 50% 16W, IGA score 0 or 1 Group A: 5% Group B:84% Group C: 53% 52W, PASI 90 Group A: NA Group B:78% Group C: 61% |
|
BE RADIANT (BKZ vs SEC) |
Group A: 373 (A1:147, A2: 215) Group B:370 |
Group A: BKZ 320mg Q4W up to 16 W, then Q4W(A1) and Q8W (A2) Group B: SEC 300mg q1w up to 4W, then Q4W |
48W |
4W, PASI 75 Group A: 71% Group B: 47.3% 16W, PASI 100 Group A: 61,7% Group B: 48.9% 48W, PASI 100 Group A: 67% Group B: 46.2% |
ADA: Adalimumab, BKZ: Bimekizumab, IGA: Investigator global assessment, PASI: Psoriasis area severity index, Q4W: Every 4 weeks, Q8W: Every 8 weeks, SEC: Secukinumab, UST: Ustekinumab, vs: Versus, W: Week
The BE COMPLETE’s study and its open-label extension BE VITAL’s 52-week phase III trial was conducted to demonstrate the efficacy and safety of bimekizumab treatment in patients with active psoriatic arthritis and prior inadequate response to tumour necrosis factor inhibitors.4 The BE MOBILE 1 and BE MOBILE 2 studies confirmed bimekizumab’s efficacy in treating patients with active axial spondyloarthritis over 52 weeks.5 BE HEARD I and BE HEARD II, two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials to assess the efficacy and safety of bimekizumab in patients with moderate to severe hidradenitis suppurativa showed promising results.6
Adverse effect and safety profile
Bimekizumab is generally well tolerated, but common side effects include upper respiratory infections, oral candidiasis, urinary tract infections, and injection site reactions [Table 2].20 One notable side effect is oral candidiasis, which is seen more frequently with bimekizumab compared to other IL-17A inhibitors like secukinumab, likely due to its dual inhibition of IL-17A and IL-17F. Most cases of candidiasis are mild and easily managed with antifungal treatment.
| TEAE | EAIR/100PYin over three years |
|---|---|
| Serious infections | 1.2 |
| Active tuberculosis | 0 |
| Fungal infections | 16.7 |
| Candidal infections | 11.5 |
| Oral candidiasis | 10.1 |
| Oropharyngeal candidiasis, skin candidiasis, adjudicated MACE | 0.6 |
| Vulvovaginal candidiasis, neutropenia | 0.4 |
| Oesophageal candidiasis, Adjudicated IBD | 0.2 |
| Tinea infection | 2.4 |
| Malignancy | 0.8 |
| Adjudicated SIB, serious hypersensitivity reaction | 0.1 |
|
AST or ALT elevation 3x ULN 5x ULN |
2.1 0.6 |
| Injection site reaction | 1.8 |
ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, BKZ: Bimekizumab, EAIR: Exposure-adjusted incidence rate, IBD: Inflammatory bowel disease, MACE: Major adverse cardiac event, PY: Patient years, SIB: Suicidal ideation, and behaviour, TEAE: Treatment-emergent adverse event, ULN: The upper limit of normal.
Long-term safety data from the BE BRIGHT study showed no new safety concerns in over three years of treatment, with adverse events either remaining consistent or decreasing with longer exposure to the drug and was lower with BKZ every eight weeks versus every four weeks.20
Conclusion
Bimekizumab is a novel biologic that offers significant advancements in the treatment of moderate to severe plaque psoriasis and related conditions. Its dual inhibition of IL-17A and IL-17F provides superior control over inflammatory pathways, leading to faster and more sustained clearance of psoriatic lesions. Clinical trial data, including off-label uses, demonstrate its versatility in managing various inflammatory disorders. For Indian patients, the emergence of bimekizumab represents an important step forward in treating severe forms of psoriasis. However, challenges such as high cost and limited access remain key hurdles. Future research should focus on long-term safety, dosing regimens, and strategies to improve accessibility for patients in India.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Psoriasis in India: Prevalence and pattern. Indian J Dermatol Venereol Leprol. 2010;76:595-601.
- [CrossRef] [PubMed] [Google Scholar]
- Bimekizumab for the treatment of psoriasis: A review of the current knowledge. Psoriasis (Auckl). 2022;12:127-37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rapid remission of plaque psoriasis with bimekizumab treatment. J Drugs Dermatol. 2024;23:694-6.
- [CrossRef] [PubMed] [Google Scholar]
- Bimekizumab treatment in patients with active psoriatic arthritis and prior inadequate response to tumour necrosis factor inhibitors: 52-week safety and efficacy from the phase III BE COMPLETE study and its open-label extension BE VITAL. RMD Open. 2024;10:e003855.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bimekizumab treatment in patients with active axial spondyloarthritis: 52-week efficacy and safety from the randomised parallel phase 3 BE MOBILE 1 and BE MOBILE 2 studies. Ann Rheum Dis. 2024;83:199-213.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): Two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials. Lancet. 2024;403:2504-19.
- [CrossRef] [PubMed] [Google Scholar]
- A case of scalp psoriasis resistant to ixekizumab treated with bimekizumab. JAAD Case Rep. 2023;38:123-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bimekizumab for the treatment of plaque psoriasis with involvement of genitalia: A 16-week multicenter real-world experience – IL PSO (Italian Landscape Psoriasis) Dermatol Pract Concept. 2024;14:e2024052.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sub-erythrodermic psoriasis successfully treated with bimekizumab: A case report. Dermatol Ther. 2022;35:e15952.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Two cases of generalized pustular psoriasis successfully treated with bimekizumab. J Dermatol. 2023;50:e357-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of severe palmoplantar pustular psoriasis with bimekizumab. JAMA Dermatol. 2024;160:199-203.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bimekizumab in refractory pityriasis rubra pilaris. J Dtsch Dermatol Ges. 2024;22:102-4.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment with bimekizumab of a psoriatic patient undergoing hemodialysis: A case report and review of the literature. J Clin Med. 2024;13:2250.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bimekizumab injection prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761151s005s006s007lbl.pdf
- Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): Efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-98.
- [CrossRef] [PubMed] [Google Scholar]
- Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): A multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-86.
- [CrossRef] [PubMed] [Google Scholar]
- Bimekizumab versus Adalimumab in Plaque Psoriasis. N Engl J Med. 2021;385:1149-50.
- [CrossRef] [PubMed] [Google Scholar]
- Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-52.
- [CrossRef] [PubMed] [Google Scholar]
- Bimekizumab maintenance of response through 3 years in patients with moderate-to-severe plaque psoriasis: Results from the BE BRIGHT open-label extension trial. Br J Dermatol. 2023;188:749-59.
- [CrossRef] [PubMed] [Google Scholar]
- Bimekizumab safety in patients with moderate-to-severe plaque psoriasis: Pooled data from up to 3 years of treatment in randomized phase III trials. Br J Dermatol. 2024;190:477-85.
- [CrossRef] [PubMed] [Google Scholar]





