Translate this page into:
Bullous pemphigoid presenting as a malar rash
Correspondence Address:
Rochelle L Castillo
Department of Medicine, Section of Dermatology Clinic, Philippine General Hospital, Taft Avenue, Manila
Philippines
| How to cite this article: Castillo RL, Silva CY, Frez ML. Bullous pemphigoid presenting as a malar rash. Indian J Dermatol Venereol Leprol 2014;80:80-82 |
Sir,
Localized bullous pemphigoid (BP) is characterized by chronic intermittent vesiculobullous eruptions affecting a restricted area of the body. [1] It constitutes only 16-29% of all BP cases and has been reported on the oral mucosa, breast, genitals and lower extremities. [2],[3],[4] No case of BP presenting as a malar rash has been reported to date.
An otherwise healthy 28-year-old Filipino female consulted for 16 months - history of recurrent eruptions of tense, non-scarring vesicles and bullae overlying a persistent urticarial plaque spanning both malar areas and the nose associated with tense bullae on the oral mucosa. There was no fever, photosensitivity, discoid rash, alopecia, joint pain, or other systemic symptoms. No other sites on the body were affected and no triggers could be identified.
Dermatologic examination revealed multiple tense bullae and erosions overlying an erythematous, blanching plaque spanning both malar areas and the nose [Figure - 1]a. Nikolsky sign was negative. Tender, tense bullae were noted on the anterior maxillary and mandibular gingival mucosa [Figure - 2]a and c. There were no lesions elsewhere. Systemic physical examination was unremarkable.
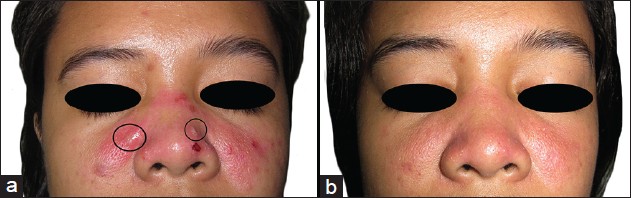 |
| Figure 1: (a) On initial examination, tense bullae (encircled), vesicles and erosions were seen overlying an erythematous, blanching plaque spanning the malar areas. (b) By week 11 of dapsone, erythema had decreased by 90% and no recurrence of vesiculobullous eruptions was noted |
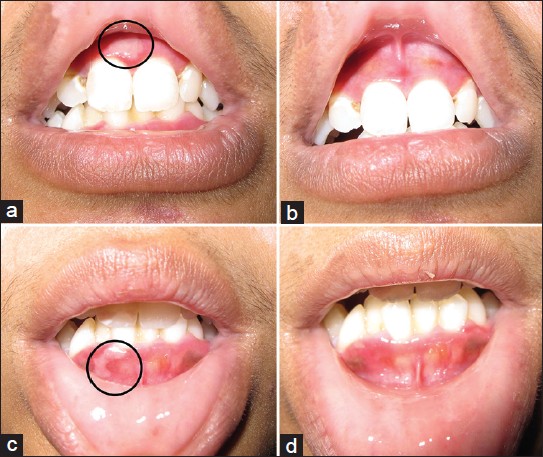 |
| Figure 2: Tense bullae (encircled) were seen on the upper (a) and lower (c) gingival mucosa. (b and d) The bullae completely resolved after 4 days of treatment |
Skin punch biopsy showed a subepidermal blister containing eosinophils and lymphocytes with a diffuse dermal infiltrate of eosinophils, lymphocytes, neutrophils and plasma cells [Figure - 3] and negative Alcian blue staining. Direct immunofluorescence (DIF) microscopy revealed a strong, continuous linear deposition of C3 along the basement membrane zone with a weak, discontinuous linear deposition of immunoglobulin G (IgG). Indirect immunofluorescence microscopy on salt-split skin and an autoantibody panel consisting of antinuclear antibodies, anti- SSA/Ro, anti-Smith and anti-double stranded deoxyribonucleic acid were negative. Based on the clinical findings of tense, non-scarring bullae overlying urticarial plaques without identifiable triggers and systemic symptoms, the histopathologic findings of a subepidermal blister with mixed infiltrates and the DIF findings of a strong, continuous linear deposition of C3 along the basal membrane zone (BMZ) with a weak linear deposition of IgG, a diagnosis of localized BP was made.
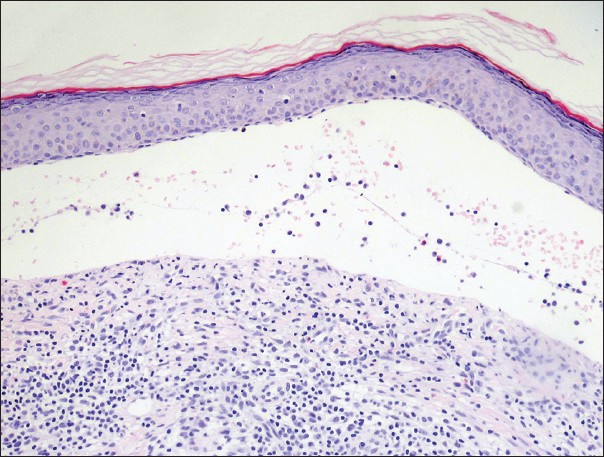 |
| Figure 3: Punch biopsy showing a subepidermal blister containing eosinophils, neutrophils and lymphocytes and a moderately dense lichenoid mixed cell infiltrate (H and E, ×20) |
The patient was given oral dapsone 100 mg once daily for 7 weeks, tapered to 50 mg daily for 4 weeks before discontinuation. Within 2 weeks of treatment, a 50% decrease in erythema of the urticarial plaque, resolution of cutaneous and oral mucosal bullae [Figure - 2]b and d and absence of new lesions were observed. By the last day of intake of dapsone 50 mg daily, the erythema had decreased by 90% and no recurrence of bullae was noted [Figure - 1]b.
To our knowledge, this is the first reported case of localized BP presenting as a malar rash. Further contributing to its uniqueness is the patient′s young age, as BP occurs mainly in the elderly. When seen exclusively on the malar areas, localized BP can be mistaken for acute cutaneous lupus erythematosus (ACLE). However, ACLE usually occurs in the setting of acutely flaring systemic lupus erythematosus, whereas our patient did not have any systemic symptoms. Our patient′s DIF findings are likewise inconsistent with ACLE, which presents with granular deposition of IgG and/or IgM at the BMZ and around hair follicles. [5] Finally, the negative autoantibody panel further rules out ACLE.
Local factors such as physical trauma, ultraviolet light, burns and hydrostatic pressure may play a role in the pathogenesis of localized BP via a phenomenon known as epitope spreading. [1],[3] Epitope spreading refers to the development of an immune response to epitopes distinct from and non-cross-reactive with, the disease-causing epitope. [6] In the context of localized BP, the epitope spreading phenomenon also applies to situations in which tissue damage from a primary inflammatory process causes the release and exposure of a previously "sequestered" antigen, leading to a secondary autoimmune response against the newly-released antigen. [7] To further correlate this with localized BP, local trauma caused by various agents (i.e. ultraviolet light, burns, or radiation) and the ensuing primary inflammatory process that this trauma incites may cause significant tissue damage, leading to disruption of the basement membrane architecture and unmasking of the BP antigens that were previously "sequestered" or "hidden" from the immune system. The unmasking of these BP antigens may subsequently lead to induction of the autoimmune process against these antigens, thereby initiating the cascade of events leading to clinically-apparent localized BP. [1],[3],[5],[8] This is supported by cases of BP arising on post-irradiation and post-surgical sites and by the relatively higher incidence of pretibial BP in females, which may be explained by increased leg exposure in women wearing skirts. [1] The presence of the lesions on our patient′s malar areas suggests the role of ultraviolet light exposure in precipitating the disease.
While topical corticosteroids constitute the first-line treatment for localized BP, dapsone may be warranted in cases of localized disease with oral mucosal involvement, as in our patient.
| 1. |
Tran JT, Mutasim DF. Localized bullous pemphigoid: A commonly delayed diagnosis. Int J Dermatol 2005;44:942-5.
[Google Scholar]
|
| 2. |
Balachandran C, Rai VM. Localised bullous pemphigoid in the breast. Indian J Dermatol Venereol Leprol 2006;72:158-9.
[Google Scholar]
|
| 3. |
Patsatsi A, Lazaridou E, Papagaryfallou I, Sotiriadis D. Bullous pemphigoid of the perineum and perianal area: A rare localized form in adults. Acta Derm Venereol 2008;88:401.
[Google Scholar]
|
| 4. |
Urano S. Localized bullous pemphigoid of the vulva. J Dermatol 1996;23:580-2.
[Google Scholar]
|
| 5. |
Bernard P, Borradori L. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3 rd ed. Philadelphia: Elsevier Saunders; 2012. p. 475-82.
[Google Scholar]
|
| 6. |
Powell AM, Black MM. Epitope spreading: Protection from pathogens, but propagation of autoimmunity? Clin Exp Dermatol 2001;26:427-33.
[Google Scholar]
|
| 7. |
Chan LS, Vanderlugt CJ, Hashimoto T, Nishikawa T, Zone JJ, Black MM, et al. Epitope spreading: Lessons from autoimmune skin diseases. J Invest Dermatol 1998;110:103-9.
[Google Scholar]
|
| 8. |
Garrett A. Localized bullous pemphigoid arising in a cholecystectomy incision site: A phenomenon of epitope spreading? J Am Acad Dermatol 2007;56:AB118.
[Google Scholar]
|
Fulltext Views
4,547
PDF downloads
3,511





