Translate this page into:
Bullous pemphigoid with malignant melanoma
2 Shandong Provincial Institute of Dermatology and Venereology, Shandong Academy of Medical Sciences, Jinan, Shandong, China
3 Department of Dermatology, Shandong Provincial Hospital of Dermatology and Venereology, Jinan, Shandong; Shandong Provincial Institute of Dermatology and Venereology, Shandong Academy of Medical Sciences, Jinan, Shandong, China
Correspondence Address:
Furen Zhang
Shandong Provincial Institute of Dermatology and Venereology, 27397 Jingshi Road, Jinan, Shandong Province
China
| How to cite this article: Parimi LR, Chen M, Liu H, Zhang F. Bullous pemphigoid with malignant melanoma. Indian J Dermatol Venereol Leprol 2015;81:625-626 |
Sir,
Bullous pemphigoid is a chronic autoimmune blistering disease that predominantly affects elderly men over 60 years but can occur in children. It is characterized by the linear deposition of immunoglobulin G (IgG) and/or C3 along the basement membrane zone, visible on direct immunofluorescence. Indirect immunofluorescence (IIF) demonstrates circulating IgG autoantibodies that bind to the epidermal side of the basement membrane zone (roof pattern) on a salt-split skin preparation. On immunoblot analysis, it is characterized by the presence of IgG autoantibodies specific for the hemidesmosomal bullous pemphigoid antigens BP230 (BPAg1) and BP180 (BPAg2). BP 180 is the usual pathogenic antibody. The association between bullous pemphigoid and malignancy is controversial.[1]
A 74-year-old Chinese man presented to the out-patient clinic of Shandong Provincial Hospital of Dermatology and Venereology, Jinan, China with oral mucosal erosions for 50 days with a 1-month history of extensive blisters all over his body. He was treated as a case of skin allergy in other hospitals. History revealed a background of depression for 6 years. His general examination was unremarkable. Cutaneous examination revealed an extensive eruption of tense bullae all over his body including the face with painful lesions in the oral cavity [Figure - 1]a and[Figure - 2]a. Histopathology showed subepidermal blisters with abundant lymphocytes and eosinophils in the underlying dermis [Figure - 1]b. Linear deposition of IgG and C3 along the basement membrane zone was observed on direct immunoflouresence [Figure - 1]c. Indirect immunoflourescence was negative for basement membrane zone antibodies. Salt-split skin demonstrated an epidermal pattern [Figure - 1]d. A diagnosis of bullous pemphigoid was made and he was treated with oral prednisone, 100 mg daily which was gradually tapered to 50 mg daily at the time of discharge, and cyclophosphamide 0.2 g intravenous drip daily, which was reduced to 0.6 g intravenous drip once a week. Marked improvement in the skin and oral mucosal lesions were noticed over a period of 35 days.
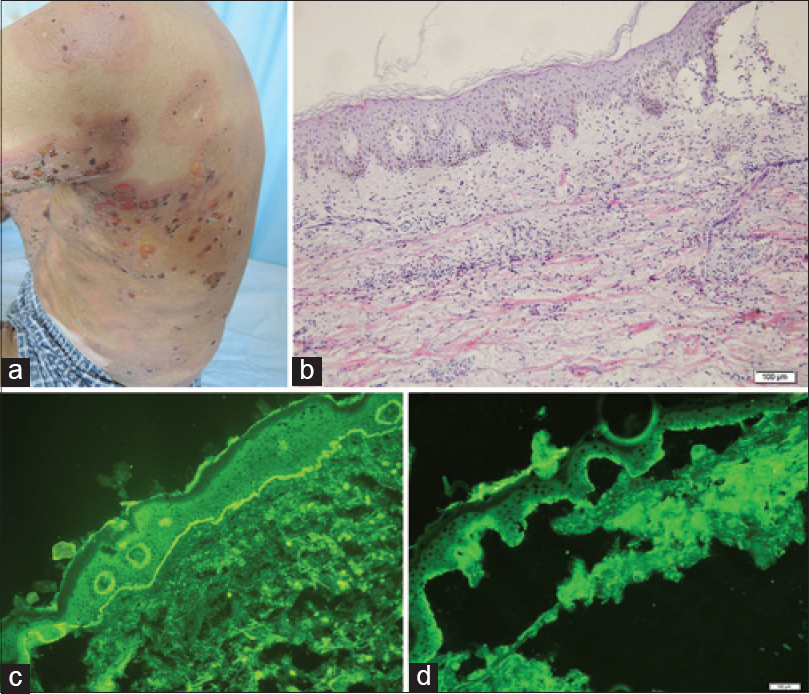 |
| Figure 1: (a) Multiple, tense bullae with erythema seen on the back. (b) Subepidermal blister with abundant lymphocytes and eosinophilic infiltrate in the underlying dermis (H and E, ×10). (c) Direct immunofluorescence showed linear deposition of immunoglobulin G and C3 along the basement membrane zone. (d) Salt-split skin substrate demonstrated an epidermal/roof pattern |
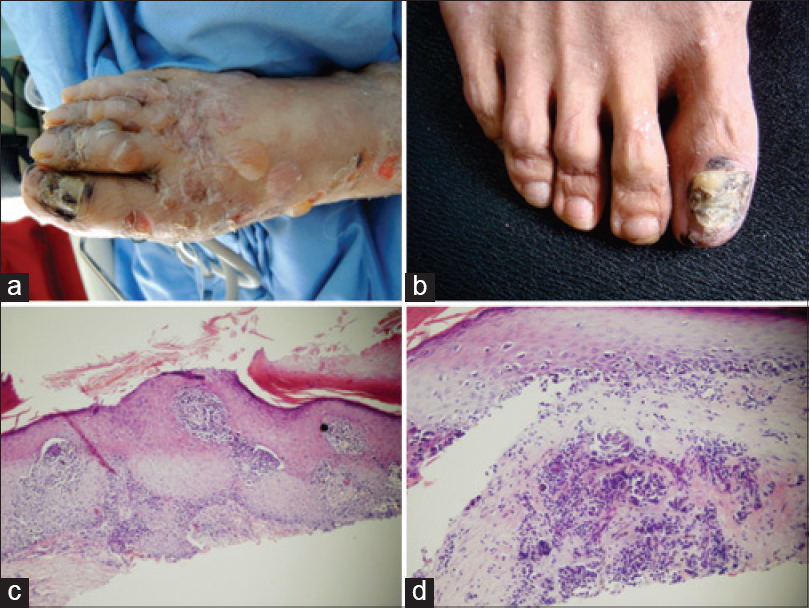 |
| Figure 2: (a) Multiple, tense bullae with erythema seen on the right foot. (b) Darkening of nail bed with nail plate dystrophy on the right toe. (c) Atypical melanocytes are seen in the epidermis and dermis. Some atypical melanocytes with surrounding halo also exist (H and E, ×20). (d) Buckshot scatter of individual melanocytes are seen in lower line of the epidermis. Melanocytes nest invaded into the basement membrane (H and E, ×40) |
During follow up, his skin lesions were stable with no recurrence of disease activity. Prednisone was tapered to 35 mg daily with cyclophosphamide 50 mg, twice daily. On his second visit, we identified darkening of the nail bed with nail plate dystrophy on his right toe [Figure - 2]b. All other toe and hand nails were normal. The patient revealed that the nail changes appeared 20 years ago and he never received any prior treatment. Biopsy from the lesion showed tumor invasion into the basement membrane with S-100, HMB 45 and Melan-A all being positive [Figure - 2]c and [Figure - 2]d. Based on the histological findings, a diagnosis of malignant melanoma was confirmed. His right toe was excised. Chest X-ray revealed relatively small intrapulmonary nodules indicating lung metastasis with lymph node involvement. He was therefore referred to the oncology hospital for further treatment.
The paraneoplastic nature of pemphigoid is controversial. There have been reports where the malignancy occurred before, during and after the appearance of typical bullous skin lesions. The precise pathogenetic mechanism of this association is unclear. Studies postulated that the antibodies produced against tumor-specific antigens might cross-react with the basement membrane zone. Other theories include the possibility that the same external agent might generate the cancer and the basement membrane zone damage or that there is a genetic predisposition to both diseases.[1] Another hypothesis suggests that the tumor may produce BP180 antigen or may expose these normally sequestered antigens. The latter is described as the phenomenon of epitope spreading where the injured mucosa or the carcinoma might expose the BP180 antigen inducing the onset of disease.[2]
Shimbo et al.8 reported anti-BPAg1 auto-antibodies as a promising marker for the diagnosis of melanoma. They identified that BPAg1 is expressed in human melanoma cell lines (A375 and G361) and normal human melanocytes.[3] Muramatsu et al. examined the antigen specificities of autoantibodies in sera from three patients with pemphigoid associated with malignancies. They stated that in all the three cases, auto-antibodies against BP180, but not against BP230, were detected.[4]
Venencie et al. noticed a higher incidence of malignant disease in pemphigoid patients with negative IIF findings.[5] Our patient also suffered atypical disease (seronegative bullous pemphigoid) suggesting that the relation between these two conditions needs further evaluation.
The association between pemphigoid and malignancy has been debated for years. Notably, these two conditions are more prevalent in the older population. Therefore, further studies identifying the significance of risk with a particular type of cancer are vital for appropriate screening and to reduce health costs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Iranzo P, López I, Robles MT, Mascaró JM Jr, Campo E, Herrero C. Bullous pemphigoid associated with mantle cell lymphoma. Arch Dermatol 2004;140:1496-9.
[Google Scholar]
|
| 2. |
Chan LS, Vanderlugt CJ, Hashimoto T, Nishikawa T, Zone JJ, Black MM, et al. Epitope spreading: Lessons from autoimmune skin diseases. J Invest Dermatol 1998;110:103-9.
[Google Scholar]
|
| 3. |
Shimbo T, Tanemura A, Yamazaki T, Tamai K, Katayama I, Kaneda Y. Serum anti-BPAG1 auto-antibody is a novel marker for human melanoma. PLoS One 2010;5:e10566.
[Google Scholar]
|
| 4. |
Muramatsu T, Iida T, Tada H, Hatoko M, Kobayashi N, Ko T, et al. Bullous pemphigoid associated with internal malignancies: Identification of 180-kDa antigen by western immunoblotting. Br J Dermatol 1996;135:782-4.
[Google Scholar]
|
| 5. |
Venencie PY, Rogers RS 3rd, Schroeter AL. Bullous pemphigoid and malignancy: Relationship to indirect immunofluorescent findings. Acta Derm Venereol 1984;64:316-9.
[Google Scholar]
|
Fulltext Views
2,619
PDF downloads
2,137





