Translate this page into:
Can etanercept be an option in management of recurrent steroid-dependent erythema nodosum leprosum? A retrospective study of six patients
Corresponding author: Dr. Suman Patra, Department of Dermatology, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. patrohere@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sahani MK, Asati DP, Kaur M, Patra S. Can etanercept be an option in management of recurrent steroid-dependent erythema nodosum leprosum? A retrospective study of six patients. Indian J Dermatol Venereol Leprol 2022;88:243-6.
Sir,
Erythema nodosum leprosum is an immune complex-mediated reaction seen in leprosy patients in lepromatous spectrum of disease.1 Systemic corticosteroids are the mainstay of treatment but are not safe for long-term use in cases of recurrent episodes. Thalidomide, an effective steroid sparing agent is beyond the reach of most of the patients due to its cost. Other immunosuppressants such as methotrexate, cyclosporine and azathioprine have been found to have variable efficacy.2-4 As tumour necrosis factor (TNF) is one of the main mediators of erythema nodosum leprosum, biologicals with tumour necrosis factor inhibition as target have a potential role. We share our experience with six cases of recurrent erythema nodosum leprosum treated with etanercept.
We retrospectively analysed the data from patients of erythema nodosum leprosum, who received injection etanercept during the years 2018–2019 in the Department of Dermatology, All India Institute of Medical Sciences, Bhopal. Patients with steroid dependent erythema nodosum leprosum of greater than six months duration who had poor control with conventional immunosuppressants for at least three months, received etanercept. The diagnosis of erythema nodosum leprosum was based on classical clinical presentation, histopathology and slit skin smear. Other medical treatments taken by patients were either continued or tapered/escalated in follow-up according to standard guidelines by NLEP. Pre treatment evaluation included complete blood count, liver function test, hepatitis B, C and HIV screening, chest X-ray and Mantoux test. Etanercept was administered every week as 50 mg subcutaneous injection one hour after premedication. Treatment response was categorised in terms of change in frequency of erythema nodosum leprosum, number of lesions in each episode, appearance of necrotic lesions and total cumulative steroid doses received six months before and after receiving treatment with etanercept. The parameters were compared using paired t-test.
A total of six male patients (age: 13–43 years) of erythema nodosum leprosum (mean BI: 3) received etanercept over a one-year period. Two patients were continuing MB-MDT (multibacillary multi drug therapy) while four others had completed the regimen prior. The duration of erythema nodosum leprosum ranged from 6 to 18 months (10 ± 4.6 months). All patients were on prednisolone 20–40 mg/day, while two patients each were additionally on methotrexate 15 mg/wk and thalidomide 100 mg/day respectively while one patient was on clofazimine 100 mg three times a day. The frequency of erythema nodosum leprosum episodes ranged from 1 to 5 (mean = 3) in the past six months and cumulative prednisolone dose ranged from 625 to 2180 mg (mean: 1489.83 mg). All the patients had necrotic lesions of erythema nodosum leprosum as well [Figure 1]. The number of lesions varied from 15 to 50 lesions/episode (mean: 37). Patients received between one to nine doses of etanercept (1–9) depending on the availability of the medicine in hospital supply and treatment response [Figure 2].

- Necrotic erythema nodosum leprosum lesions over arm in a patient
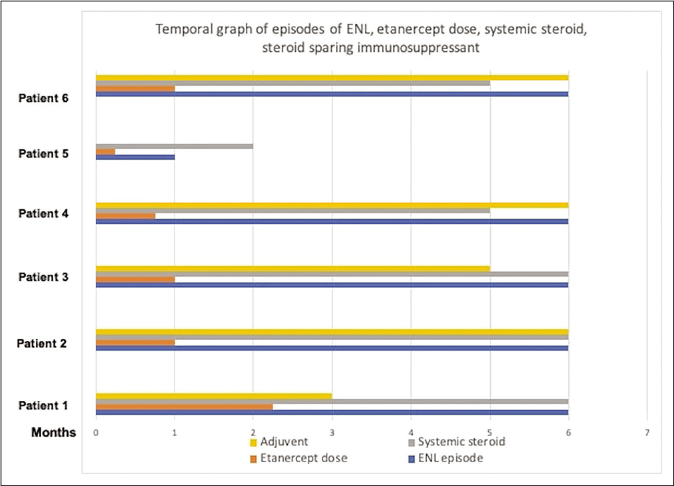
- Temporal graph of erythema nodosum leprosum episodes, etanercept doses, systemic steroid and steroid sparing immunosuppressant in the six months after starting etanercept
All patients showed improvement in erythema nodosum leprosum after treatment. One patient did not develop any further erythema nodosum leprosum episodes after the first dose itself, three out of the remaining five had remission while on treatment but relapsed on stopping etanercept. Another two patients continued to develop erythema nodosum leprosum episodes but with lesser severity during treatment period. The frequency of erythema nodosum leprosum (number of episodes in every six months) decreased from mean of 2.67 to 2 during next six months (P = 0.174). There was 42.8% decrease in cumulative prednisolone dose from a mean of 1489.8mg before etanercept to 851.5mg after receiving etanercept (P = 0.03). The average number of erythema nodosum leprosum lesions at each episode decreased significantly, from a mean of 37 to 16 (P = 0.019) [Table 1] [Figure 3]. None of the patients developed necrotic lesions post-treatment. In three patients steroids could be stopped completely within six months, while in the remaining three tapering of the prednisolone dose was possible.
| Serial no | Age/sex | Duration (months) | Severity of erythema nodosum leprosum in last six months | Etanercept dose | Time to response | Effect at six months | Adverse events | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency/six months | Morphology | No. of lesions /episode |
Previous treatment | Other signs | Cumulative prednisolone dose in milligram (mg) | ||||||||
| 1 | 40/M | 10 | 2 | Necrotic | 50 | Prednisolone, Aspirin, Clofazamine | Fever, arthralgia |
1950 | 50 mg, 3 dose |
One week | No necrotic lesion and less constitutional symptoms Decrease in frequency of episodes: 1 and number of lesions (20) Cumulative prednisolone requirement: 1400 mg | Weight gain |
|
| 2 | 43/M | 18 | 2 | Necrotic | 50 | Methotrexate, prednisolone, thalidomide, aspirin, pregabalin | Neuritis, fever, arthralgia | 2180 | 50 mg, 4 dose |
One week | No necrotic lesion and less constitutional symptoms Prednisolone stopped Increase in frequency of episodes: 3, same number of lesions (50) Cumulative prednisolone requirement: 952 mg | Weight gain, dyspepsia | |
| 3 | 39/M | 6 | 3 | Necrotic | 35 | Thalidomide, prednisolone | Neuritis, fever, arthralgia | 2325 | 50 mg, 9 dose |
One week | No necrotic lesion and less constitutional symptoms Frequency of episodes: same: 3, decrease in no. of lesions (10) Cumulative prednisolone requirement: 975 mg |
Weight gain, pyoderma | |
| 4 | 30/M | 6 | 3 | Necrotic | 50 | Prednisolone, clofazimine, aspirin | Neuritis, fever, arthralgia | 975 | 50 mg, 4 dose |
One week | No necrotic lesion and less constitutional symptoms Prednisolone stopped Decreased frequency of episodes: 1 and number of lesions (10) Cumulative prednisolone requirement: 450 mg | Weight gain |
|
| 5 | 28/M | 8 | 1 | Necrotic | 15 | Prednisolone | Neuritis, fever, arthralgia | 884 | 50 mg, 1 dose |
One week | No necrotic lesion and less constitutional symptoms Prednisolone stopped Decreased frequency of episodes: 0, no lesions Cumulative prednisolone requirement: 922 mg | Weight gain |
|
| 6 | 13/M | 12 | 5 | Necrotic | 20 | Prednisolone, thalidomide | Neuritis, fever, arthralgia | 625 | 25 mg, 4 dose |
One week | No necrotic lesion and less constitutional symptoms Decreased frequency of episodes: 4 and no. of lesions (10) Cumulative prednisolone requirement: 410 mg | Weight gain, pyoderma | |
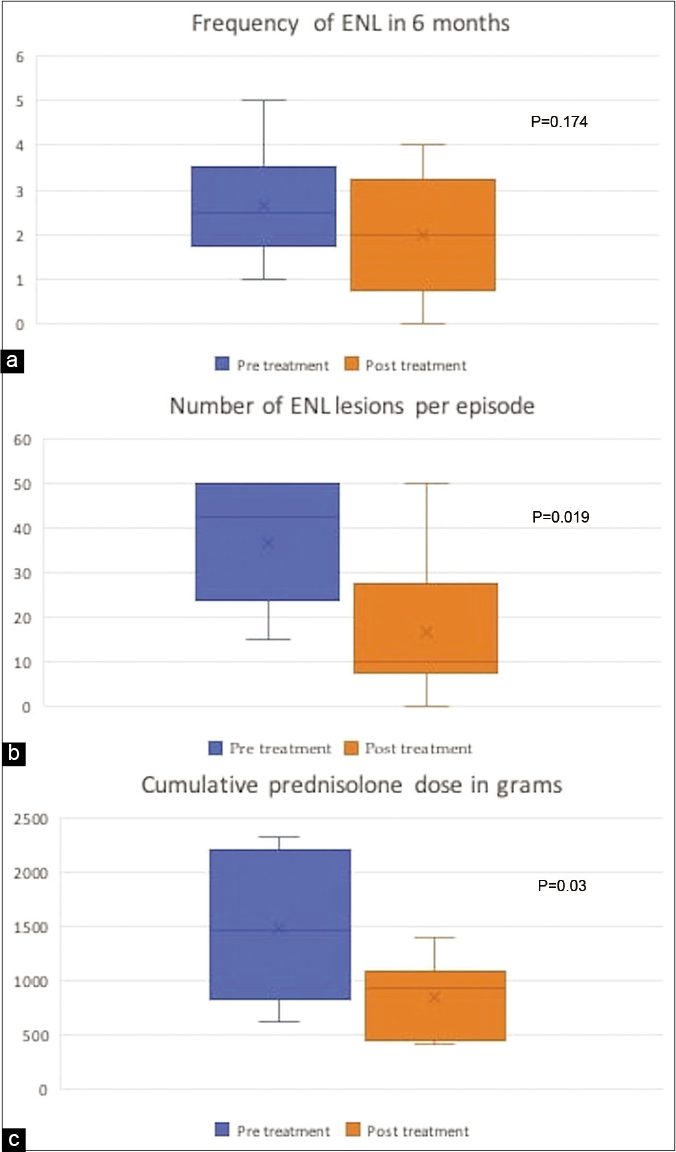
- Change in frequency of erythema nodosum leprosum: (a) average number of lesions per episode (b), cumulative steroid dose and (c) before and after treatment with etanercept
All the patients experienced minor side effects in the form weight gain (6), dyspepsia (1) and pyodermas (2). However, none of the patients experienced injection site reactions or any serious adverse event.
Erythema nodosum leprosum is an immune complex mediated reaction where tumour necrosis factor is considered as the main mediator.5,6 Tumour necrosis factor inhibitor biologicals have been reported to be effective in management of erythema nodosum leprosum in few reports.7-11 Faber et al. showed good and sustained response in one patient of erythema nodosum leprosum following three doses of infliximab.8 We chose etanercept due to its easy availability in our hospital low treatment cost. Etanercept has been used previously in steroid dependent erythema nodosum leprosum in isolated case reports showing beneficial role allowing tapering and discontinuation of systemic steroids. However, duration of treatment varied from six weeks to 12 months. In contrast, we found significant reduction in frequency and severity of erythema nodosum leprosum episodes along with decrease in total cumulative steroid doses of steroid with much fewer doses [Figure 3]. The limitation of our study was its retrospective study design and variability in dosing. No standard protocol was followed for treatment. Based on this study alone, it cannot be said with certainty that etanercept is a treatment option for erythema nodosum leprosum. However, it reflects that etanercept can be considered for future randomised controlled trials in erythema nodosum leprosum to prove its actual efficacy as a steroid sparing agent in this condition.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Leprosy Type 1 reactions and erythema nodosum leprosum. An Bras Dermatol. 2008;83:75-82.
- [CrossRef] [Google Scholar]
- Comparison of efficacy and safety of ciclosporin to prednisolone in the treatment of erythema nodosum leprosum: Two randomised, double blind, controlled pilot studies in Ethiopia. PLoS Negl Trop Dis. 2016;10:e0004149.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine in chronic recalcitrant erythema nodosum leprosum: A case report. J Clin Diagn Res. 2017;11:FD01-2.
- [Google Scholar]
- Using methotrexate to treat patients with ENL unresponsive to steroids and clofazimine: A report on 9 patients. Lepr Rev. 2013;84:105-12.
- [Google Scholar]
- New insights in the pathogenesis of Type 1 and Type 2 lepra reaction. Indian J Dermatol Venereol Leprol. 2013;79:739-49.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin Exp Immunol. 1991;84:103-8.
- [CrossRef] [Google Scholar]
- Treatment of recurrent erythema nodosum leprosum with infliximab. N Engl J Med. 2006;355:739.
- [CrossRef] [PubMed] [Google Scholar]
- Severe refractory erythema nodosum successfully treated with the tumor necrosis factor inhibitor etanercept. Clin Infect Dis. 2011;52:e133-5.
- [CrossRef] [PubMed] [Google Scholar]
- Biologics in leprosy: A systematic review and case report. Am J Trop Med Hyg. 2020;102:1131-6.
- [CrossRef] [PubMed] [Google Scholar]
- Etanercept in erythema nodosum leprosum. An Bras Dermatol. 2017;92:575-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of severe refractory erythema nodosum leprosum with tumor necrosis factor inhibitor Etanercept. Int J Mycobacteriol. 2016;5:223-5.
- [CrossRef] [PubMed] [Google Scholar]





