Translate this page into:
Chemotherapy-induced reticulate pigmentation in three Indian patients including a case in the pediatric age group
Corresponding author: Dr. Keshavamurthy Vinay, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh - 160 012, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar S, Bhattacharjee R, Kambhampati SB, Narang T, Kanwar AJ, Vinay K. Chemotherapy induced reticulate pigmentation in three Indian patients including a case in pediatric age group. Indian J Dermatol Venereol Leprol 2021;87:386-8.
Sir,
Pigmentary changes of skin, hair and nails are well-acknowledged adverse effects of cancer chemotherapy. Several patterns of chemotherapy-induced cutaneous hyperpigmentation have been described, namely diffuse (busulfan, cyclophosphomide), palmoplantar (5-fluorouracil, ifosfamide, tegafur), flagellate (bleomycin) and supravenous (5-fluorouracil) and at the sites of trauma or friction (cyclophosphamide, fluorouracil, thiotepa).1,2 Chemotherapy-induced reticulate pigmentation is a rare entity with only a few cases described in the literature.3,4 We were unable to find any report either in the pediatric age group or in Indian patients. Herein, we report three cases of chemotherapy-induced reticulate pigmentation in patients of Indian ethnicity, including a pediatric one.
The index case was a nine-year-old boy suffering from high-risk T-cell acute lymphoblastic leukemia (ALL), on consolidation phase treatment with methotrexate, cyclophosphamide, cytarabine, vincristine and asparaginase. One month after initiating the consolidation phase, he developed asymptomatic, net like, macular, brown to black hyperpigmented lesions (without any other surface changes like scaling or ulceration) on lower back, buttocks and back of thighs [Figure 1]. The lesions resolved spontaneously three months after stopping the chemotherapy.
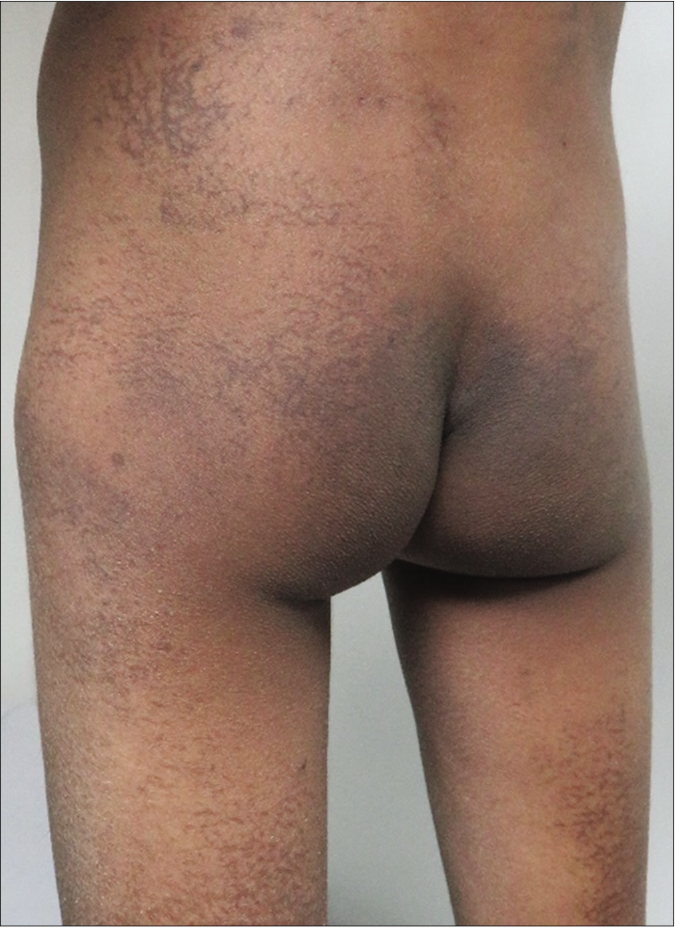
- Chemotherapy-induced reticulate pigmentation. Net like macular brown to black hyperpigmented lesions without other surface changes like scaling or ulceration on lower back, buttocks and posterior thigh in the index case
The second patient was a 20-year-old male, also a case of T-cell ALL on treatment with rituximab, methotrexate and cytarabine, who developed asymptomatic reticulate pigmentation on upper back and side of neck [Figure 2] within a week of starting chemotherapy. The lesions resolved spontaneously four months after cessation of chemotherapy.
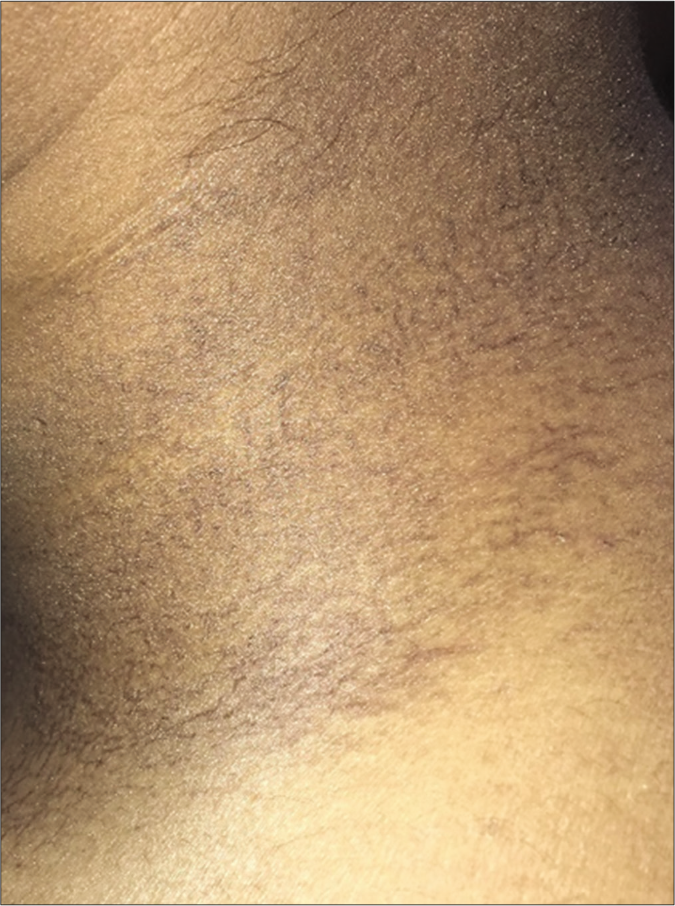
- Net like macular brown to black hyperpigmented lesions without other surface changes like scaling or ulceration on neck of case 2
The third patient was an elderly male in his seventies who developed asymptomatic reticulate pigmented lesions [Figure 3] primarily over his back within two weeks of starting chemotherapy with carboplatin and gemcitabine for hepatocellular carcinoma. The patient is still undergoing chemotherapy and the lesions are persisting.
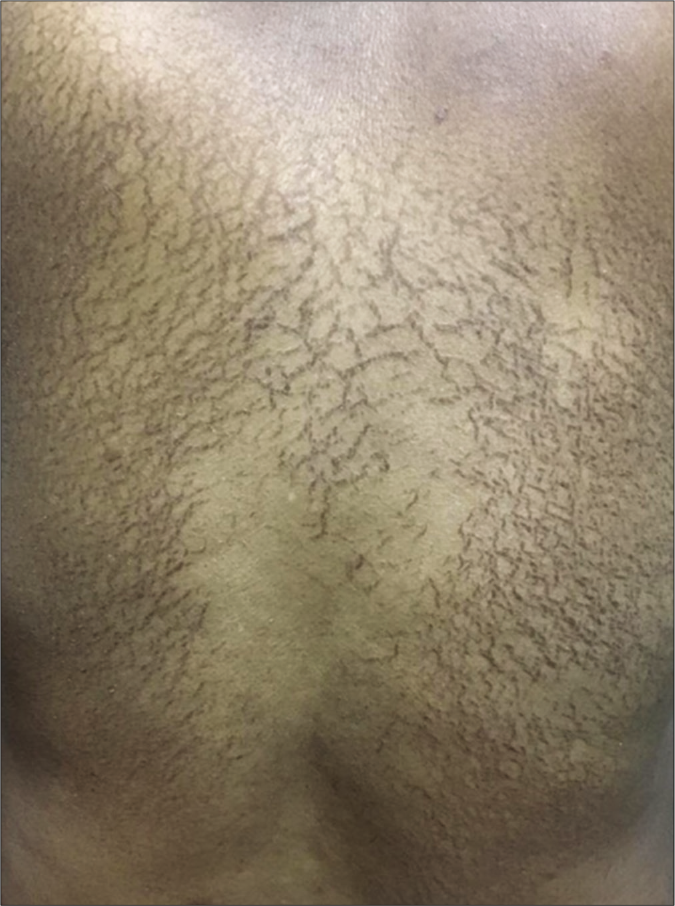
- Net like macular brown to black hyperpigmented lesions without other surface changes like scaling or ulceration on back of case 3
A diagnosis of chemotherapy-induced reticulate pigmentation was reached in all the above patients based on the temporal association of onset of reticulate pigmentation with the initiation of chemotherapy. Given the benign and self-limiting nature of this adverse effect, no active intervention was done in any of the three patients.
Drug-induced reticulate pigmentation is a rare entity and has been reported with oral diltiazem, topical benzoyl peroxide, regional intravenous anesthesia with prilocaine and chemotherapeutic agents.3 First described by Wright et al. in 1990,5 several chemotherapeutic agents including cyclophosphamide, ifosfamide, 5-fluorouracil, paclitaxel, idarubicin, cytarabine have been implicated to cause chemotherapy-induced reticulate pigmentation.3,4 However, most of the patients developing the condition in previous reports were on multiple chemotherapeutic agents and implicating a specific drug was not possible.4
Clinically the lesions present as lacy and/or net-like macular brown to black colored hyperpigmentation without any surface changes like scaling or ulceration. Back is the most commonly involved site followed by legs, buttocks, shoulder and abdomen. Mild pruritus or pre-existing erythema can be associated in few cases. The hyperpigmentation usually fades in 2 to 6 months after stopping chemotherapy and the patient should be counseled on the benign nature of this adverse effect.3,4
This is the first report of chemotherapy-induced reticulate pigmentation in the pediatric age group and Indian patients. The clinical presentation and course in our pediatric patient was same as adults with resolution of lesions after stopping the treatment. The first two patients were on regimen containing cytarabine which is known to cause this condition. The third patient was receiving carboplatin and gemcitabine and carboplatin in combination with 5-flurouracil has been reported to cause reticulate pigmentation in a woman with mucoid epidermoid carcinoma of the parotid gland.4
To conclude, chemotherapy-induced reticulate pigmentation is a rare benign cutaneous adverse effect of chemotherapeutic agents and the patients and clinicians should be aware of its self-limiting course.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Drug-induced skin pigmentation. Epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous drug Reactions: Chemotherapy-induced hyperpigmentation. Eur J Dermatol. 2017;27:679-80.
- [CrossRef] [PubMed] [Google Scholar]
- Chemotherapy-related reticulate hyperpigmentation: A case series and review of the literature. Dermatology. 2015;231:312-8.
- [CrossRef] [PubMed] [Google Scholar]
- Paclitaxel-associated reticulate hyperpigmentation: Report and review of chemotherapy-induced reticulate hyperpigmentation. World J Clin Cases. 2016;4:390-400.
- [CrossRef] [PubMed] [Google Scholar]
- Reticulate pigmentation due to bleomycin: light-and electron-microscopic studies. Dermatologica. 1990;180:255-7.
- [CrossRef] [PubMed] [Google Scholar]





