Translate this page into:
Childhood vitiligo
Correspondence Address:
Arun C Inamadar
Department of Dermatology, Venereology and Leprosy, Sri B.M. Patil Medical College, Hospital and Research Center, BLDE University, Bijapur - 586 103, Karnataka
India
| How to cite this article: Palit A, Inamadar AC. Childhood vitiligo. Indian J Dermatol Venereol Leprol 2012;78:30-41 |
Abstract
Childhood vitiligo is often encountered in dermatological practice. When present in infancy or early childhood, various nevoid and hereditary disorders are to be differentiated. In many cases, familial aggregation of the disease is seen and other autoimmune disorders may be associated. Segmental presentation is more common, and limited body surface area involvement is usual in this age group. Children with vitiligo often suffer from anxiety and depression because of their unusual appearance. Management of vitiligo in children is difficult as therapeutic options are restricted when compared to that in adult patients. Selection of treatment should be careful in these patients with the aim to achieve best results with minimal side effects as well as relieving patients' and parents' anxiety.Introduction
Vitiligo is an acquired pigmentary disorder occurring irrespective of age, sex and race. The most important aspect of vitiligo is the cosmetic concern it arouses in the psyche of patients and their family members because of the stigma associated with it. This is more so among dark races for the obviousness of the disease.
Vitiligo may present anytime in life, including the neonatal period and childhood. Childhood vitiligo deserves special attention as frequently (50%), the disease onset is before 20 years of age and, in 25% of the cases, it starts before the age of 10 years. [1] In general, childhood vitiligo differs from the adult disease in the following aspects: a female preponderance is observed, segmental presentation is more common and associated other autoimmune or endocrine disorders are rarer. [2]
Classification
In most of the epidemiological studies, childhood vitiligo has been categorized as "segmental" and "non-segmental" types. Segmental vitiligo (SV) implies occurrence of depigmented macules and patches along dermatomal or quasi-dermatomal pattern, without crossing the midline [Figure - 1]. [3] In non-segmental vitiligo (NSV), the skin lesions may be generalized (vitiligo vulgaris, universal vitiligo) or localized (focal, mucosal, acrofacial, acral). Vitiligo vulgaris implies widely scattered depigmented lesions, whereas almost-total depigmentation of skin is termed as universal vitiligo. [2] Focal vitiligo is the occurrence of one or few depigmented lesions localized to one body area not corroborating to a dermatome [Figure - 2]. [2] Acral vitiligo is confined to the distal extremities [Figure - 3]; in combination with facial lesion it is of the acrofacial type, and mucosal vitiligo involves one or multiple mucosae. [2]
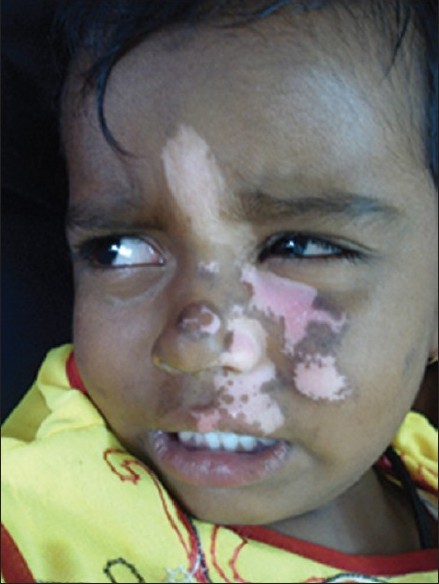 |
| Figure 1: Segmental vitiligo over the face |
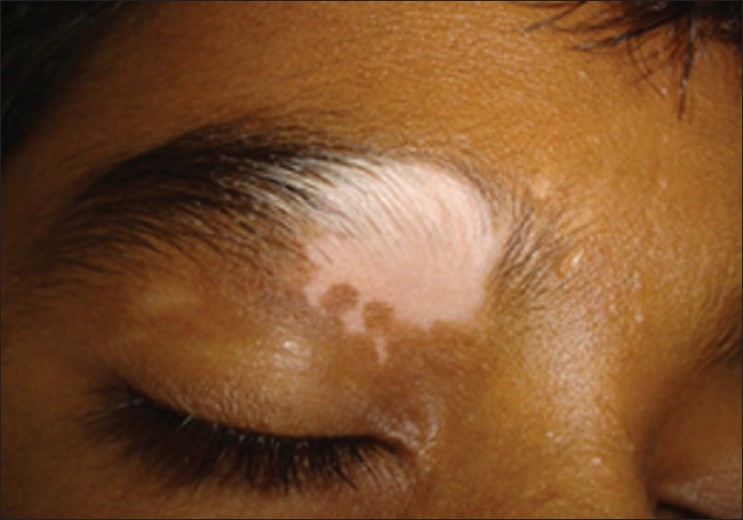 |
| Figure 2: Focal vitiligo with leukotrichia |
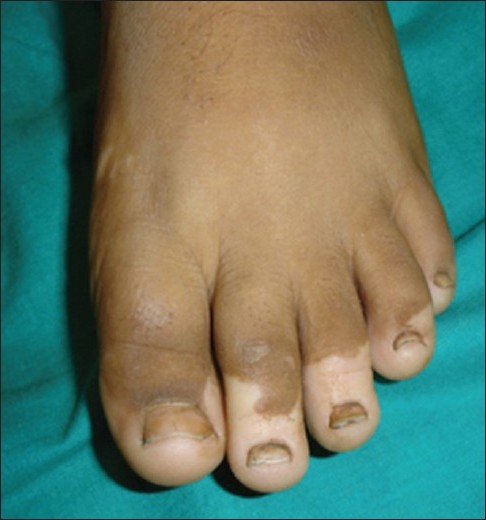 |
| Figure 3: Acral vitiligo in a child |
Pathogenesis
Detailed discussion on the pathogenesis of vitiligo is beyond the scope of this article. Recent advances in this aspect of vitiligo have been discussed in brief.
Genetic susceptibility in vitiligo is evidenced by frequent clustering in families and occurrence in monozygotic twins. Various susceptibility loci (autoimmunity susceptibility gene) for vitiligo are AIS1 (chromosome 1), AIS2 (chromosome 7), AIS3 (chromosome 8) and SLEV1 (chromosome 17). [4] Association of vitiligo with "transporter associated with antigen processing protein-1 (TAP-1)" gene is suggestive of the possible role of MHC class I antigen in antimelanocyte autoimmune response. [5] Studies on HLA class I and class II genes in vitiligo have revealed strong association and negative association with certain HLA types.
Various hypotheses prevail regarding the pathogenesis of NSV. These include autoimmune mechanism, autocytotoxic hypothesis, aberration of cellular immunity with melanocyte destruction, inhibition of melanogenesis and aberration of vitamin D3 metabolism. [4] Autoimmune mechanism for NSV and neural hypothesis for SV are most explanatory. [4]
NACHT-leucine-rich-repeat protein-1 (NALP1) gene (chromosome 17p13) confers to the occurrence of a group of multiorgan autoimmune and autoinflammatory disorders, including vitiligo. [6] Mutation in the autoimmune regulator (AIRE) gene (chromosome 21q22.3) results in a rare recessive disorder, "autoimmune polyendocrinopathy candidiasis ectodermal dystrophy syndrome (APECED)." [5] Vitiligo is more than 10-times common in patients with APECED compared with the general population. [5] Link to NALP1 and AIRE gene may explain the association of vitiligo to other autoimmune disorders and presence of circulating autoantibodies in these patients. [5]
There is wide expression of NALP1 in all tissues, especially at a high level in T cells and Langerhans′ cells. NALP1 forms a complex termed "NALP1 inflammasome" by the recruitment of adapter protein ASC, caspase 1 and caspase 5. [6] NALP1 inflammasome activates proinflammatory cytokine interleukin-1b, which has been found to be raised in generalized vitiligo. [6] Hence, this pathway might be involved in the pathogenesis of vitiligo.
Epidemiology
In various studies, the prevalence of vitiligo in childhood (age <12 years) has been quoted to be around one quarter of vitiligo patients of all ages. In a Chinese study, 24.1% of the vitiligo patients were in the pediatric age group. [7] Among Korean patients with vitiligo, 16% were children. [8] In two Indian studies, the prevalence has been reported to be 26% (south India) [9] and 23.3% (north India), [10] respectively. The prevalence of SV is higher in children (17-29%) as compared to that in adults (5%). [8] Among the various ethnic groups, SV is more common in the Korean population. [8] Cho et al[8] have reported 32.5% of SV in their series of Korean children.
Onset of the disease is usually below 10 years of age. The Mean age at onset of childhood vitiligo in an Indian study was 6.2 years; [10] the same was 5.6 years and 7.28 years in Korean and Chinese studies, respectively. [7],[8] SV presents earlier, may be soon after birth. [3] Hann et al[3] have reported disease onset below 10 years of age among 41.3% of their series of patients with SV. In a Chinese study on childhood vitiligo (n = 541), eight children had skin lesions present at birth (focal 7, acrofacial 1). [7]
An Indian study [10] has quoted a statistically significant difference in the occurrence of vitiligo among boys and girls, but few other studies have not recorded such a difference.
Family members of the affected children have a higher incidence of vitiligo and other autoimmune disorders compared to controls. [2] Positive family history in childhood vitiligo varies between 11% [7] and 46% [11] in various studies. [7],[8],[11] Pajvani et al[2] have reported an earlier onset of vitiligo in children in whom family history of the disease, leukotrichia or other autoimmune disorders were present. In children with focal and segmental disease, family history of vitiligo or other autoimmune disorders is usually negative. [2],[12]
Clinical Features
Vitiligo is characterized by asymptomatic, well-demarcated, ivory-white macules and patches that may be localized or generalized.
Any of the clinical variants of vitiligo may occur in childhood. Vitiligo vulgaris is the most common clinical type observed in various clinical studies, followed by focal vitiligo and SV. [7],[8],[10],[11] Acrofacial and mucosal vitiligo have a lower incidence in childhood. Different studies have quoted variable incidence of mucosal vitiligo in children; Halder et al[13] 0%, Handa et al[10] 0.6% and Jaisankar et al[9] 13.8%. Of the mucosal sites, oral mucosal vitiligo is rarer in children as compared to adults. [4] The rarest type seen during childhood is the universal vitiligo.
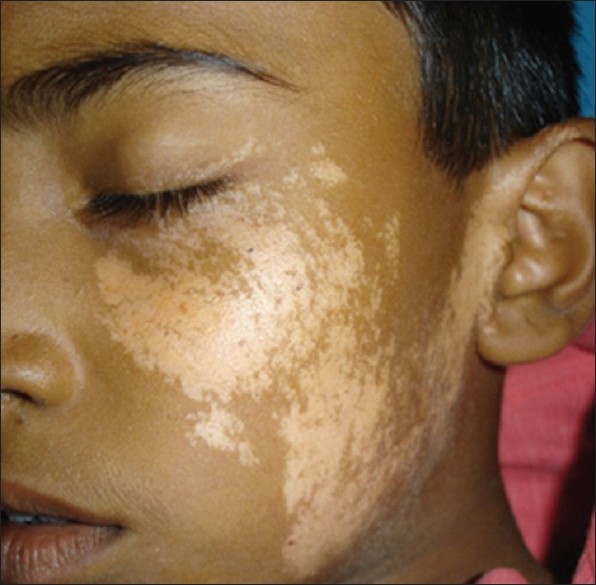
Common initial site of onset of both NSV and SV in children is the face and neck. [3] In NSV, initial lesions are periocular, perinasal or perioral, and gradually spread to other body parts, in a more or less symmetrical manner. Perineum, perianal area and, in infancy, the diaper area may be the initial site of occurrence of skin lesions [Figure - 4]. [4] Individual vitiligo macules may enlarge attaining a geographic pattern or there may be appearance of new lesions at other sites. Although extensive areas of depigmentation may be present, majority of the children have <20% body surface area (BSA) involvement. [4],[10] Focal vitiligo may subsequently evolve into generalized disease.
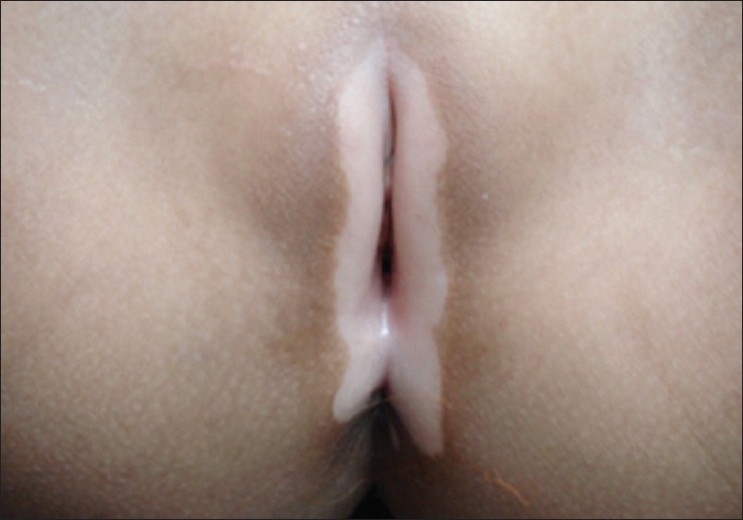 |
| Figure 4: Genitalia as the site of onset in childhood vitiligo |
In the series of patients with SV studied by Hann et al, [3] trigeminal segment was the most common dermatome involved, followed by thoracic, cervical, lumbar and sacral. Majority of the children in this series (87%) had a single lesion. [3]
In white-skinned children, vitiligo lesions may remain unapparent initially and may become evident for the first time following suntan during a holiday. [4] In dark-skinned children, typical multishaded trichrome patches may be present. Mazereeuw-Hautier et al[14] have recorded the occurrence of a hyperpigmented rim around the depigmented lesions only among the children with NSV (8.99%).
Koebner phenomenon may be noted in both SV and NSV in children, more commonly in the latter type. In SV, Koebnerization is confined to the involved segment only. Koebnerization may be more frequent in childhood vitiligo because of higher mobility and playfulness in this age group. Presence of Koebnerization is indicative of the disease activity.
Cho et al[8] have reported scalp involvement in 25% of the children with vitiligo. Scalp leukotrichia, defined as "significant extent of graying of scalp hair without underlying vitiliginous area before 30 years of age," [3] is a frequent finding in children with vitiligo and their family members. Poliosis, defined as a localized patch of white hair, [15] is commonly seen in patients with vitiligo, more so in SV. [11] Prcic et al[11] observed poliosis among 55.55% of children and Hann et al[3] among 48.6% of the patients with SV. Eyebrows were the most commonly involved sites. [3]
Associations
Vitiligo may be associated with other autoimmune disorders like alopecia areata, diabetes mellitus, pernicious anemia, Addison′s disease and thyroid disorder. An Indian study has quoted associated autoimmune disorders in 1.3% of the children with vitiligo. [10] Several authors have reported vitiligo-associated autoimmune disorders occurring exclusively in children suffering from NSV. [1],[14] Mazereeuw-Hautier et al[14] have reported associated thyroid function abnormalities without clinical disease in 11.23% of the children with NSV but in none with SV. In the study by Hann et al,[3] associated autoimmune disorders were found in 3.4% of the children with SV.
Among adult patients with vitiligo, autoimmune thyroiditis (Hashimoto′s) resulting in hypothyroidism is more common (30%) [16] as compared to the general population (10%). [12]
Lacovelli et al[1] have studied the relevance of thyroiditis and other autoimmune diseases in 121 pediatric patients with vitiligo. The significant findings of this study were as follows: [1]
- Sixteen percent of the children with NSV showed altered thyroid function parameters, but none among the children with SV.
- Altered thyroid parameters were more common in girls than in boys.
- Hypothyroidism was the more common association of childhood vitiligo as compared to hyperthyroidism, in the ratio of 6:1.
Authors of this study have stressed upon routine thyroid screening in pediatric patients with vitiligo as diagnosis of autoimmune thyroiditis is particularly important in this age group to avoid the negative impact of hypothyroidism on growth and health status. [1] Kakourou et al[17] have reported an occurrence of autoimmune thyroiditis among 24.1% of children and adolescents with vitiligo. In this study, female preponderance was observed among adolescents (>12 years) with vitiligo and autoimmune thyroiditis (F:M = 6:1) when compared to children (F:M = 1:2). [17] The authors have suggested that this gender predilection for adolescent females may be estrogen mediated. [17] In majority of the cases, skin lesions of vitiligo precede the clinical features of thyroid dysfunction. [17] Rarely, occurrence of thyroid autoantibodies may precede the appearance of vitiligo lesions. [4],[17]
Other autoimmune disorders are also more common in NSV. The reported disorders in association with childhood vitiligo are alopecia areata, celiac disease, [17],[18] diabetes mellitus, [1],[10] polyglandular autoimmune syndrome, [10] Addison′s disease [10] and pemphigus vulgaris. [10] Rodrνguez-Garcνa et al[18] have reported a 9-year-old girl with vitiligo vulgaris unresponsive to conventional therapy. Subsequently, she was diagnosed to have celiac disease and institution of gluten-free diet resulted in progressive repigmentation of her skin lesions by 1 year, which continued even after 7 years without any active treatment for vitiligo. [18] Atopic disorders, urticaria, Down syndrome and uveitis may be associated. [1],[3]
Although associated endocrinopathies and autoimmune disorders are lower in childhood vitiligo as compared to adults, asymptomatic occurrence of autoantibodies are more common in this age group. [19]
Often, anomalous laboratory parameters may be the only finding. Some authors have reported altered thyroid function tests (10.74%), [1] positive anti-gastric parietal cell antibodies (0.8%) [1] and positive antinuclear antibody (4.8%) in children with vitiligo. [13] Incidence of antinuclear antibody positivity is lower in children with vitiligo as compared to that in adults. [11]
Vitiligo-associated dermatosis, like halo nevi, occurs commonly in children, the incidence in various studies varying from 2.5% to 34%. [7],[8],[10],[11] These occur more commonly in children with NSV.
Course of the Disease
The course of childhood vitiligo is mostly stable or regressive; only few patients experience progressive or recurrent disease. [2] Complete spontaneous repigmentation of NSV is unusual. However, as compared to adults, the rate of spontaneous repigmentation is more in children, especially in tropical countries and during summer months. [20] Repigmentation may be diffuse, marginal or perifollicular. Following the initial onset, SV spreads fast only along the affected dermatome. Thereafter, it remains stationary for the rest of the patient′s life. [3] In a large series of patients of all ages suffering from SV, progression was seen among 55.3% of patients, lesions were stable in 40.9% patients and minimal regression without treatment was seen in 3.8% of patients. [3]
Differential Diagnosis
Various nevoid and hereditary disorders with depigmentation may simulate vitiligo in children. It is important to differentiate hereditary disorders from early-onset childhood vitiligo as these are usually multisystemic. Moreover, therapeutic intervention is possible in vitiligo but not feasible in nevoid and hereditary conditions. [Table - 1] presents the list of various congenital and acquired conditions simulating vitiligo and the important differentiating features [Figure - 5]. [20]
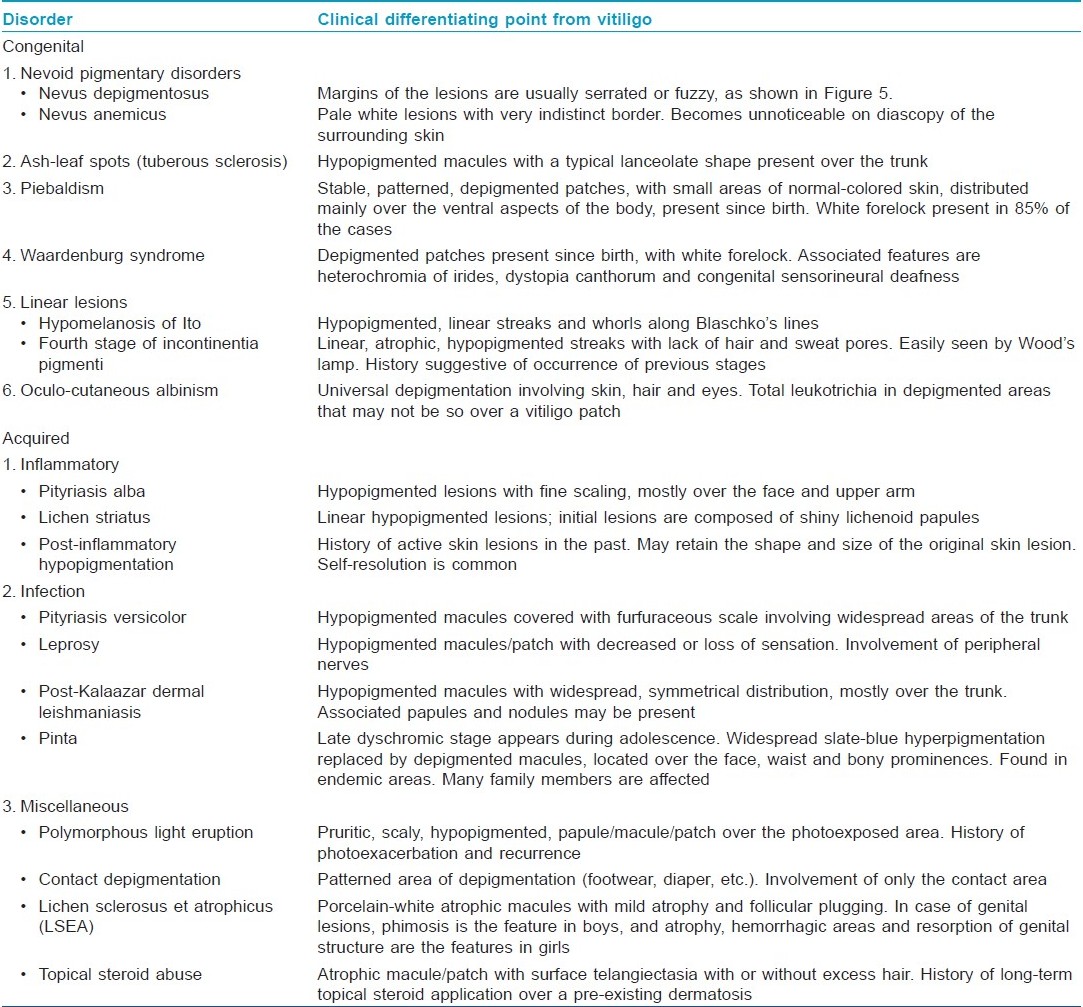
 |
| Figure 5: Serrated margin of nevus depigmentosus |
Vitiligo as a component of hereditary syndromes
Vitiligo in older children and adolescents may be one of the components of certain syndromes. [20]
Vogt-Koyanagi-Harada syndrome: It is a rare syndrome affecting children, especially of south-east Asian origin. Characteristic features are uveitis, aseptic meningitis, dysacusia, alopecia, poliosis and vitiligo. Uveitis is the presenting feature and vitiligo may appear later, during the chronic stage (fourth stage) of the disease (adolescence or adulthood). Vitiligo lesions tend to be symmetrical, involving the head, neck and trunk. The sacral region is a common site of involvement with vitiligo. Poliosis may involve the scalp, eyebrows and eyelashes.
Alezzandrini syndrome: This syndrome is characterized by SV (cheek), poliosis, ipsilateral uveitis resulting in decreased visual acuity and same-sided partial hearing loss. Manifestation starts during adolescence.
In both these disorders, uveitis and related ocular manifestations are the main clinical features. Vitiligo appears later and is usually persistent, despite therapy.
Management
Diagnosis
Diagnosis of vitiligo is mostly clinical. Invasive and sophisticated investigations are not required to confirm the diagnosis. In children with fair skin, it may be difficult to differentiate a lesion of vitiligo from the surrounding normal skin. In these cases, examination under Wood′s lamp is helpful. [20] Complete blood count and fasting blood sugar should be performed as a routine work-up for all patients. In case of diagnostic difficulty, skin biopsy may be taken; histopathological examination shows total absence of melanocytes in established lesions of vitiligo. In early lesions, melanocytes are still retained but with multiple abnormalities like vacuolization, dilated endoplasmic reticulum and granular deposits. [4] Presence of mild inflammatory infiltrate is sometimes seen, and is indicative of disease activity. [4]
Associated autoimmune disorders should be ruled out in the pediatric age group. Screening for autoantibodies may be performed if facilities are available. Antinuclear antibody may be positive even in normal children. Thyroid function status of the child is assessed by estimating T3, T4 and TSH levels. Coexistence of autoimmune thyroiditis may be ruled out by the estimation of antithyroglobulin antibody (anti-Tg) and antithyroperoxidase antibody (anti-TPO). Kakourou et al[17] have proposed a management protocol for children with vitiligo and positive antithyroid antibodies [Figure - 6].
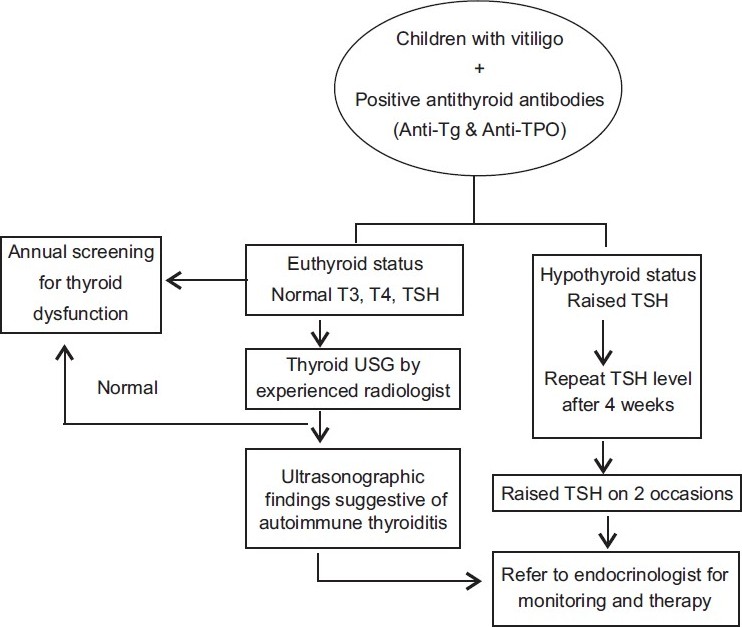 |
| Figure 6: Proposed protocol for the investigation and management of children with vitiligo and positive antithyroid antibodies[17] |
Therapy
Various therapeutic modalities are available for the treatment of vitiligo; however, all of these cannot be used in children. Medical therapy is considered as the first line of management in this age group. All children with widespread vitiligo should undertake photoprotection preferably with opaque sunscreens during daytime outdoor activities. In general, localized vitiligo is treated with topical therapy. Widespread or generalized disease is managed with phototherapy or systemic therapy. Surgical methods may be chosen for stable (size of the lesion is stationary for > 2 years and no new lesion has developed recently) [21] localized vitiligo and SV. Different therapeutic options available for the treatment of childhood vitiligo are presented in [Table - 2]. [22] Certain clinical features are poor prognostic markers for treatment. These include acral vitiligo, presence of leukotrichia over vitiliginous area and lesions over bony prominences like elbow, knee and ankle. An algorithm for treatment options in childhood vitiligo has been presented in [Figure - 7].

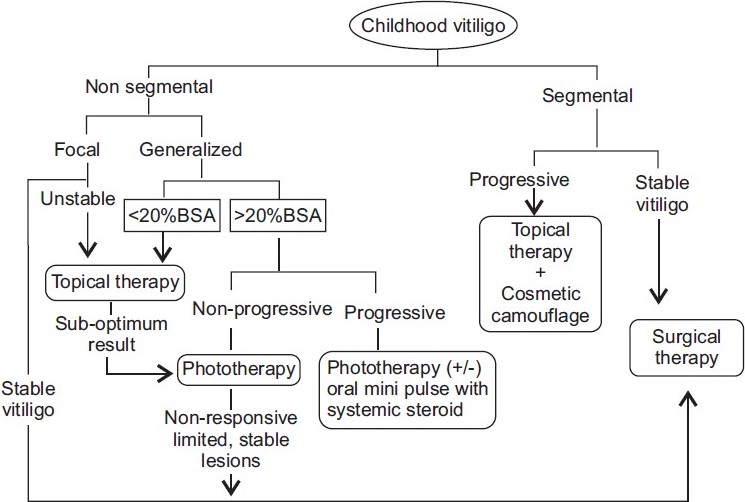 |
| Figure 7: Algorithm presenting the treatment options for childhood vitiligo |
Medical treatment
Topical therapy
Mid-potent topical corticosteroids are the first-line therapy for children with localized vitiligo. Although high-potency steroids are more effective in vitiligo, these are not recommended for use in children. Available studies report a 45-60% response rate to topical steroid in childhood vitiligo. [8],[19] It requires long-term therapy for several months, increasing the chances of developing tachyphylaxis and local side effects like atrophy, telangiectasia, hypertrichosis and striae. There are risks of serious side effects like glaucoma (prolonged application on periorbital vitiligo), suppression of hypothalamic-pituitary-adrenal axis and growth retardation (long-term use over large BSA). Hence, parents should be cautioned about the inadvertent use of topical steroids.
Topical calcineurin inhibitors (TCI), tacrolimus and pimecrolimus are effective alternatives to topical corticosteroids in terms of avoidance of side effects of the latter. Several authors have demonstrated the effectiveness of tacrolimus in children with vitiligo. [23],[24],[25] In a double-blind randomized placebo-controlled trial of topical tacrolimus (0.1%) versus topical clobetasol propionate (0.05%) in childhood vitiligo (age 2-16 years), the efficacy of both the therapeutic agents was comparable and no significant adverse effect was recorded in either group. [26]
In an open comparative trial of mometasone cream (0.1%, once-daily application) and pimecrolimus cream (1%, twice-daily application) in the treatment of localized childhood vitiligo, the repigmentation rates were 65% and 42%, respectively, at the end of 3 months. [27] Mometasone cream was found to be equally effective in all body parts whereas pimecrolimus was effective only over the face. [27]
TCIs are slower to exert beneficial effect compared to topical corticosteroid. [28] Best response is observed on the thinnest areas of the skin (eyelids). [28] As the skin barrier function is not compromised in children with vitiligo (unlike atopic dermatitis), highest strength (0.1%) of tacrolimus may be used safely. [28] Calcineurin inhibitors are not yet approved for the treatment of vitiligo and are not recommended for use in children <2 years. [22]
Topical calcipotriol has been found to be effective in the treatment of vitiligo in children and adolescents. In a trial of topical calcipotriol in childhood vitiligo, repigmentation was recorded as early as 4 weeks of treatment. [29] Some interesting observations made in this study were: repigmentation of untreated lesions, continuation of repigmentation in both treated and untreated lesions even after stoppage of calcipotriol and response in previously treatment-resistant lesions (range 21.18-93.46%). These findings led the author to hypothesize that a systemic effect of topically applied calcipotriol might have been operative. [29] Combination therapy of topical corticosteroids and calcipotriol [30] may be used to reduce the side effects of corticosteroids as well as to enhance the efficacy of calcipotriol as the latter may not be as effective when used as monotherapy. [22]
Side effects of calcineurin inhibitors and calcipotriol are mild and transient and hence, easily tolerated by children. However, both these agents are more expensive compared to topical corticosteroid, and may not be easily affordable by the families from the low socioeconomic strata.
Recalcitrant cases of widespread vitiligo or universal vitiligo with only few islands of normal skin may be considered for total depigmentation therapy with 20% monobenzyl ether of hydroquinone (MBEH), with an advice of life-long stringent photoprotection. [20],[22]
Systemic therapy
Rapidly progressive generalized vitiligo in older children and adolescents may be treated with a short course of systemic steroid. A better way to avert the side effects is administering oral betamethasone as a single morning dose (0.1 mg/kg body weight) on two consecutive days in a week (oral mini-pulse therapy) for 12 weeks, and thereafter reducing the dosage by 1 mg/month for the next 3 months. [31] Pasricha et al[32] have studied the effect of oral mini-pulse therapy (OMP) in children and adults with extensive or fast-spreading vitiligo, and have observed a 26-50% response in 25%, 51-75% response in 7.5% and >75% response in 15% of patients. Phototherapy may be added to such regimen. [31] Majid et al[33] have used methylprednisolone OMP in combination with topical fluticasone for 6 months in 400 children with progressive vitiligo. Complete halt of progression was noted in >90% of the children after initiation of therapy, and >65% of children had well to excellent repigmentation at the end of the study period. [33]
Phototherapy and photochemotherapy
Systemic photochemotherapy with psoralen-ultraviolet A (PUVA) is contraindicated in small children and can be used only beyond 12 years of age. It is used when >20-25% of the BSA is involved or in patients in whom other treatment modalities are not giving optimum result. [22] Topical PUVA can be used even in younger children with limited BSA involvement. Various authors have reported an overall response rate of approximately 75% in 50-60% of the children with vitiligo treated with topical or oral PUVA therapy. [22]
Oral supplementation of L-phenylalanine (a precursor of tyrosine in melanin synthesis) and UVA phototherapy have been found to be effective in children with extensive vitiligo (50-100% repigmentation in 69% of children). [34]
Narrow-band ultraviolet B (NB-UVB) phototherapy is an effective modality for the treatment of generalized childhood vitiligo with >20% BSA involvement. [22] There are several studies on the effectiveness of NB-UVB phototherapy in children. [34],[35],[36] Brazzelli et al[37] have reported this therapeutic modality as effective and safe in children. Of the 10 children with vitiligo included in this trial, better therapeutic response was noted in those with recent-onset disease (duration <1 year). [37] Best repigmentation occurred on skin lesions over the face and neck, and moderate response was seen in lesions on the trunk and proximal extremities. Lesions over the acral region (fingers and toes), bony prominences and less-hairy areas (wrist, ankle and joints) were poorly repigmented. [37] The side effects recorded were minimal erythema, amenable to emollients and topical steroid. [37] Other topical therapeutic agents like pimecrolimus and pseudocatalase may be used in combination with NB-UVB phototherapy. [22] In a large series of children and adolescents with vitiligo, Schallreuter et al[38] have recorded a significantly better repigmentation rate (>75%) with the use of combined treatment of pseudocatalase and NB-UVB as compared to
NB-UVB alone. Rath et al[31] have compared the efficacy of combined phototherapy (PUVA, NB-UVB and broad band UVB) and OMP with OMP alone in 86 patients (aged 10-50 years) with progressive vitiligo. Combination therapy was found to be superior to OMP alone, the most effective being with NB-UVB, followed by PUVA and broad band UVB.
Excimer laser (308 nm)/targeted UVB phototherapy has been used in the treatment of localized childhood vitiligo with the advantage of more focused therapy at the site of lesion, avoiding the risk of photoageing of the surrounding skin. [22] Cho et al[39] have treated 30 children with localized vitiligo (40 patches) with 308 nm excimer laser. A repigmentation rate of >50-75% was recorded in this trial, especially over the face, neck and trunk. Side effects related to this mode of therapy are usually minimal and transient. Hui-Lan et al[40] have studied the effect of 308 nm excimer laser and topical pimecrolimus cream (1%) compared to excimer laser alone in 49 Chinese children with NSV. The effect of this combination therapy was statistically superior to the laser therapy alone, and this effect was most evident on facial lesions. [40]
Repigmentation following phototherapy is either marginal or perifollicular [Figure - 8]. During phototherapy, genitalia of the children should always be protected. [41] It is preferable to follow the "skin-saving principle," i.e. shielding of uninvolved body sites during phototherapy. [41] Already repigmented body parts should also be covered with clothing during further therapy. [41] Carcinogenic potential of NB-UVB phototherapy in children is not yet determined. [42] However, theoretically, there may be an increased chance of developing long-term skin cancers because
of the higher number of expected years of life in children. [42] Such risk may be lower in children with darker skin types. In resource-poor countries, the facility of phototherapy is available mostly in tertiary health care centers. Hence, it may not be easily accessible to families from remote localities. Moreover, multiple hospital visits confer loss of school days of children and working days of parents, reducing compliance to treatment. [42]
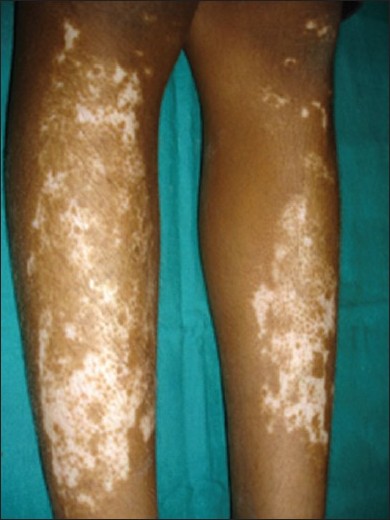 |
| Figure 8: Perifollicular repigmentation following narrow-band ultraviolet B phototherapy |
Surgical therapy
Only stable localized vitiligo lesions (segmental or non-segmental), unresponsive to other treatment modalities, are chosen for surgical treatment. Surgical procedures are not performed in very young children. This is because segmental or stable focal lesions in younger children extends proportionate to their body growth. Moreover, success of many surgical procedures depends upon the post-operative immobility of the operated part, which becomes difficult to maintain in young children. Older children and adolescents may be counseled about the procedure and the possible outcome of the surgery to achieve their cooperation. The restrictive factors for surgical procedures are inability to treat larger area and the risk of Koebnerization of the donor site.
Among the various surgical techniques, suction blister epidermal grafting (SBEG) has been found to be most convenient and effective for children and adolescents. [22] Gupta et al[43] have reported >75% repigmentation in 86.7% patches in 80% children and adolescents with vitiligo following the SBEG technique. The success rate of this technique is better in children as compared to adults. [43] However, this procedure requires prolonged immobility to facilitate smooth generation of the blisters, more so in children because of their strong dermoepidermal adherence; hence, it may be a difficult task to keep children in restricted posture till the required duration. [43]
Surgical procedures in combination with medical therapy have been tried in childhood vitiligo. In a randomized, placebo-controlled trial using microdermabrasion and pimecrolimus cream (1%) in childhood NSV, >50% repigmentation was observed after 3 months in 60.4% of patches treated with this combination as compared to pimecrolimus alone (32.1%) and placebo (1.7%). [44]
Non-cultured autologous epidermal transplantation has been used successfully in children and adolescents with stable vitiligo. Mulekar et al[45] have used this technique in 25 children with focal vitiligo and SV with follow-up till 4 and 4.5 years, respectively. Seven children with focal vitiligo (n = 12) and eight children with SV (n = 13) had near-complete to complete repigmentation with this technique. [45] Sahni et al[46] have used this technique to treat stable vitiligo lesions in 13 children and adolescents. Repigmentation rate at the end of 1 year in these patients varied from 75% to >90%.
Cultured melanocyte transplantation is a relatively tedious technique requiring specialized set up, trained staff and a preparation time of 6-8 weeks. [47] Cost may be a restrictive factor for poor families in availing this treatment modality. [47]
Cosmetic camouflage
Good-quality cover-up cosmetics (available in commercial names; Dermablend, Covermark, Dermacolor) may be used to cover localized vitiligo lesions over exposed body parts in children. [20],[22] Well-counseled older children may use this as an effective adjunctive while on treatment or when failed to respond to other treatment modalities. White-skinned children in whom conventional treatment is contraindicated or ineffective may be managed with stringent photoprotection and cosmetic camouflage.
Treatment of vitiligo in hereditary disorders
Vitiligo lesions in hereditary disorders like Vogt-Koyanagi-Harada syndrome and Alezzandrini syndrome are usually resistant to treatment. These patients would be on long-term systemic corticosteroid and various immunosuppressive agents for the treatment of uveitis. Topical steroid may be used for localized lesions of vitiligo. Sunscreens should be used regularly and cosmetic camouflage may be used. Phototherapy should be used with great caution in these patients as this may enhance ocular inflammatory disease. [48]
All children with vitiligo, especially among dark races, require thorough counseling as they may be the victims of peer-teasing and avoidance at schools. This may lead to anxiety, introvert personality and childhood depression. In a qualitative psychosocial development survey of children with vitiligo, Schwartz et al[49] have recorded a higher frequency of fear to strangers and predominant fear and shyness to a change in close relative in them as compared to their healthy siblings. The child′s perception about the illness and difficulties in interaction with other children should be discussed in detail during routine follow-up. [4] Parents of affected children should also be counseled regarding tackling such issues. A well-counseled child with vitiligo may render full cooperation to the treating physician, making his job easier. Mulekar et al[45] have observed that all the children with localized vitiligo in their series, treated by non-cultured cellular grafting technique, accepted the treatment procedure willingly, even if it was a repeat session.
Childhood vitiligo requires special consideration. Many of the adults with vitiligo had disease onset during the first or early second decades of life and had grown up with the psychological trauma associated with this stigmatizing disease. Early institution of medical care (therapy and/or counseling) in all cases of childhood vitiligo ensures better cope up with the associated stress as well as understanding the nature and course of the disease during adolescence and adulthood.
Treatment of vitiligo at any age remains a challenge for clinicians, more so during childhood. None of the available therapies is absolutely effective, and the disease runs a relapsing course. With any of the treatment modalities, >75% repigmentation (achieved by approximately 60% of treated children) is considered as the best therapeutic response. [22],[41] Long-term treatment requirement is the rule with chance of cumulative side effects of the drugs. Some patients (15-30%) remain unresponsive to all treatment [22] and even in responsive cases, there are treatment-resistant body sites. Often, multiple therapeutic modalities may have to be used to obtain optimum result in a given patient. The art of treatment of childhood vitiligo is a fine balance between addressing all these issues and achieving the best result out of the available modalities.
| 1. |
Lacovelli P, Sinagra JL, Vidolin AP, Marenda S, Capitanio B, Leone G, et al. Relevance of thyroiditis and of other autoimmune diseases in children with vitiligo. Dermatology 2005;210:26-30.
[Google Scholar]
|
| 2. |
Pajvani U, Ahmad N, Wiley A, Levy RM, Kundu R, Mancini AJ, et al. The relationship between family medical history and childhood vitiligo. J Am Acad Dermatol 2006;55:238-44.
[Google Scholar]
|
| 3. |
Hann SK, Lee HJ. Segmental vitiligo: Clinical findings in 208 patients. J Am Acad Dermatol 1996;35:671-4.
[Google Scholar]
|
| 4. |
Mazereeuw-Hautier J, Harper J. Vitiligo. In: Harper J, Oranje A, Prose N, editors. Textbook of pediatric dermatology. 2 nd ed. Oxford: Blackwell Publishing Ltd; 2006. p. 1041-56.
[Google Scholar]
|
| 5. |
Ahnini T, Mcdonagh AJ, Wengraf DA, Lovewell TR, Vasilopoulos Y, Messenger AG, et al. The autoimmune regulator gene (AIRE) is strongly associated with vitiligo. Br J Dermatol 2008;159:591-6.
[Google Scholar]
|
| 6. |
Jin Y, Mailloux CM, Gowan K, Riccardi SI, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Eng J Med 2007;356:1216-25.
[Google Scholar]
|
| 7. |
Hu Z, Liu JB, Ma SS, Yang S, Zhang XJ. Profile of childhood vitiligo in China: An analysis of 541 patients. Pediatr Dermatol 2006;23:114-6.
[Google Scholar]
|
| 8. |
Cho S, Kang HC, Hahm JH. Characteristics of vitiligo in Korean children. Pediatr Dermatol 2000;17:189-93.
[Google Scholar]
|
| 9. |
Jaisankar TJ, Baruah MC, Garg BR. Vitiligo in children. Int J Dermatol 1992;31:621-3.
[Google Scholar]
|
| 10. |
Handa S, Dogra S. Epidemiology of childhood vitiligo: A study of 625 patients from north India. Pediatr Dermatol 2003;20:207-10.
[Google Scholar]
|
| 11. |
Prcic S, Djuran V, Mikov A, Mikov I. Vitiligo in children. Pediatr Dermatol 2007;24:666.
[Google Scholar]
|
| 12. |
Hegedus L, Heidenheim M, Gervil M, Hjalgrim H, Hoier-Madsen M. High frequency of thyroid dysfunction in patients with vitiligo. Acta Derm Venereol 1994;74:120-3.
[Google Scholar]
|
| 13. |
Halder RM, Grimes PE, Cowan CA, Enterline JA, Chakrabarti SG, Kennedy JA. Childhood vitiligo. J Am Acad Dermatol 1987;16:948-54.
[Google Scholar]
|
| 14. |
Mazereeuw-Hautier J, Bezio S, Mahe E, Bodemer C, Eschard C, Viseux V, et al. Segmental and nonsegmental childhood vitiligo has distinct clinical characteristics: A prospective observational study. J Am Acad Dermatol 2010;62:945-49.
[Google Scholar]
|
| 15. |
Messenger AG, de Berker DA, Sinclair RD. Disorders of hair. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's textbook of dermatology. 8 th ed. Oxford: Wiley-Blackwell; 2010. p. 66.1-66.100.
th ed. Oxford: Wiley-Blackwell; 2010. p. 66.1-66.100.'>[Google Scholar]
|
| 16. |
Kemp EH, Waterman EA, Weetman AP. Autoimmune aspects of vitiligo. Autoimmunity 2001;34:65-77.
[Google Scholar]
|
| 17. |
Kakourou T, Kanaka-Gantenbein C, Papadopoulou A, Kaloumenou E, Chrousos GP. Increased prevalence of chronic autoimmune (Hashimoto's) thyroiditis in children and adolescents with vitiligo. J Am Acad Dermatol 2005;53:220-3.
[Google Scholar]
|
| 18. |
Rodríguez-García C, González-Hernández S, Pérez-Robayna N, Guimerá F, Fagundo E, Sánchez R. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol 2011;28:209-10.
[Google Scholar]
|
| 19. |
Halder RM. Childhood vitiligo. Clin Dermatol 1997;15:899-906.
[Google Scholar]
|
| 20. |
Paller AS, Mancini AJ. Disorders of pigmentation. In: Paller AS, Mancini AJ, editors. Hurwitz clinical pediatric dermatology. 3 rd ed. Philadelphia: Elsevier Saunders; 2006. p. 265-305.
[Google Scholar]
|
| 21. |
Gupte PD, Shenoi SD, Mutalik S. Principles of surgical management: Patient selection: Surgical modalities available: which way to go? In: Saraf V, Fernandez R, Sarangi K, editors. Vitiligo, a monograph and color atlas. Mumbai: Fulford (India) Limited; 2000.p.64-70.
[Google Scholar]
|
| 22. |
Tamesis ME, Morelli JG. Vitiligo treatment in childhood: A state of the art review. Pediatr Dermatol 2010;27:437-45.
[Google Scholar]
|
| 23. |
Grimes PE, Soriano T, Dytoc MT. Topical tacrolimus for repigmentation of vitiligo. J Am Acad Dermatol 2002;47:789-91.
[Google Scholar]
|
| 24. |
Kanwar AJ, Dogra S, Parsad D. Topical tacrolimus for treatment of childhood vitiligo in Asians. Clin Exp Dermatol 2004;29:589-92.
[Google Scholar]
|
| 25. |
Silverberg NB, Lin P, Travis L, Farley-Li J, Mancini AJ, Wagner AM, et al. Tacrolimus ointment promotes repigmentation of vitiligo in children: A review of 57 cases. J Am Acad Dermatol 2004;51:760-6.
[Google Scholar]
|
| 26. |
Ho N, Pope E, Weinstein M, Greenberg S, Webster C, Krafchik BR. A double-blind randomized placebo-controlled trial of topical tacrolimus 0.1% versus clobetasol propionate 0.05% in childhood vitiligo. Br J Dermatol 2011;165:626-32.
[Google Scholar]
|
| 27. |
Köse O, Arca E, Kurumlu Z. Mometasone cream versus pimecrolimus cream for the treatment of childhood localized vitiligo. J Dermatolog Treat 2010;21:133-9.
[Google Scholar]
|
| 28. |
Gelmetti C, Frasin A, Restano L. Innovative therapeutics in pediatric dermatology. Dermatol Clin 2010;28:619-29.
[Google Scholar]
|
| 29. |
Sarma N. Topical calcipotriol in childhood vitiligo: an Indian experience. Int J Dermatol 2004;43:856-9.
[Google Scholar]
|
| 30. |
Travis LB, Silverberg NB. Calcipotriene and corticosteroid combination therapy for vitiligo. Pediatr Dermatol 2004;21:495-8.
[Google Scholar]
|
| 31. |
Rath N, Kar HK, Sabhnani S. An open labeled, comparative clinical study on efficacy and tolerability of oral minipulse of steroid (OMP) alone, OMP with PUVA and broad / narrow band UVB phototherapy in progressive vitiligo. Indian J Dermatol Venereol Leprol 2008;74:357-60.
[Google Scholar]
|
| 32. |
Pasricha JS, Khaitan BK. Oral mini-pulse therapy with betamethasone in vitiligo patients having extensive or fast-spreading disease. Int J Dermatol 1993;32:753-7.
[Google Scholar]
|
| 33. |
Majid I, Masood Q, Hassan I, Khan D, Chisti M. Childhood vitiligo: Response to methylprednisolone oral minipulse therapy and topical fluticasone combination. Indian J Dermatol 2009;54:124-7.
[Google Scholar]
|
| 34. |
Schulpis CH, Antoniou C, Michas T, Strarigos J. Phenylalanine plus ultraviolet light: Preliminary report of a promising treatment for childhood vitiligo. Pediatr Dermatol 1989;6:332-5.
[Google Scholar]
|
| 35. |
Njoo MD, Bos JD, Westerhof W. Treatment of generalized vitiligo in children with narrow-band (TL-01) UVB radiation therapy. J Am Acad Dermatol 2000;42:245-53.
[Google Scholar]
|
| 36. |
Kanwar AJ, Dogra S. Narrow band UVB for the treatment of generalized vitiligo in children. Clin Exp Dermatol 2005;30:332-6.
[Google Scholar]
|
| 37. |
Brazzelli V, Prestinari F, Castello M, Bellani E, Roveda E, Barbagallo T, et al. Useful treatment of vitiligo in 10 children with UV-B narrow band (311nm). Pediatr Dermatol 2005;22:257-61.
[Google Scholar]
|
| 38. |
Schallreuter KU, Krüger C, Würfel BA, Panske A, Wood JM. From basic research to the bedside: efficacy of topical treatment with pseudocatalase PC-KUS in 71 children with vitiligo. Int J Dermatol 2008;47:743-53.
[Google Scholar]
|
| 39. |
Cho S, Zheng Z, Park YK, Roh MR. The 308-nm excimer laser: A promising device for the treatment of childhood vitiligo. Photodermatol Photoimmunol Photomed 2011;27:24-9.
[Google Scholar]
|
| 40. |
Hui-Lan Y, Xiao-Yan H, Jian-Yong F, Zong-Rong L. Combination of 308-nm excimer laser with topical pimecrolimus for the treatment of childhood vitiligo. Pediatr Dermatol 2009;26:354-6.
[Google Scholar]
|
| 41. |
Njoo MD, Westerhof W, Bos JD, Bossuyt PM. The development of guidelines for the treatment of vitiligo. Arch Dermatol 1999;135:1514-21.
[Google Scholar]
|
| 42. |
Dogra S, De D. Update on photo (chemo) therapy in childhood dermatoses. In: Inamadar AC, Palit A, editors. Advances in pediatric dermatology. 1 st ed. New Delhi: Jaypee Publishers; 2011. p. 146-60.
[Google Scholar]
|
| 43. |
Gupta S, Kumar B. Epidermal grafting for vitiligo in adolescents. Pediatr Dermatol 2002;19:159-62.
[Google Scholar]
|
| 44. |
Farajzadeh S, Daraei Z, Esfandiarpour I, Hosseini SH. The efficacy of pimecrolimus 1% cream combined with microdermabrasion in the treatment of non-segmental childhood vitiligo: A randomized placebo-controlled study. Pediatr Dermatol 2009;26:286-91.
[Google Scholar]
|
| 45. |
Mulekar SV, Al Aisa A, Delvi MB, Al Issa A, Al Saeed AH. Childhood vitiligo: A long term study of localized vitiligo treated by non-cultured cellular grafting. Pediatr Dermatol 2010;27:132-6.
[Google Scholar]
|
| 46. |
Sahni K, Parsad D, Kanwar AJ. Noncultured epidermal suspension transplantation for the treatment of stable vitiligo in children and adolescents. Clin Exp Dermatol 2011;36:607-12.
[Google Scholar]
|
| 47. |
Gupta S, Kumar B. Epidermal grafting in vitiligo: Influence of age, site of lesion and type of disease on outcome. J Am Acad Dermatol 2003;49:99-104.
[Google Scholar]
|
| 48. |
Janniger CK, James WD. Alezzandrini syndrome. Available from: http://www.emedicine.medscape.com/article/1117255. [accessed on 2011 Aug 6].
[Google Scholar]
|
| 49. |
Schwartz R, Sepúlveda JE, Quintana T. Possible role of psychological and environmental factors in the genesis of childhood vitiligo. Rev Med Chil 2009;137:53-62.
[Google Scholar]
|
Fulltext Views
40,433
PDF downloads
5,104





