Translate this page into:
Chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) in psoriasis
2 Departments of Dermatology, INHS Asvini, Mumbai, Maharashtra, India
3 Departments of Medicine, INHS Asvini, Mumbai, Maharashtra, India
Correspondence Address:
J Sridhar
INHS Kalyani, Gandhigram, Visakhapatnam 530 005, Andhra Pradesh
India
| How to cite this article: Sridhar J, Desylva P, Singh Y D. Chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) in psoriasis. Indian J Dermatol Venereol Leprol 2006;72:133-135 |
Abstract
Background: Insights into the pathogenesis of psoriasis have provided opportunities to target key steps in the disease process. Tumor necrosis factor-alpha (TNF-a) being crucial to the pathogenesis of psoriasis, monoclonal antibodies against this cytokine have proved useful in its treatment. Aim: To study the efficacy of chimeric monoclonal antibody to TNF-a (infliximab) in Indian patients with recalcitrant psoriasis vulgaris. Materials and Methods: Three patients with recalcitrant psoriasis vulgaris were studied. Baseline haemogram, biochemical parameters, chest radiograph and Mantoux skin test were performed. A loading dose regimen of 5 mg/kg infliximab was administered at weeks 0, 2 and 6. PASI assessment, adverse drug event monitoring and laboratory assessments were carried out at 2-week intervals until week 10. Patients were followed up until week 22 for relapse. Results: Infliximab was well tolerated. The mean PASI was 25.4 at presentation and declined to 5.5 at 10 weeks. PASI 75 was attained at a mean of 9.6 weeks. Relapse occurred at a mean of 18.6 weeks after the first infusion. Conclusions: This study on Indian patients brings out the importance of cytokine-based therapies in psoriasis. Indigenous production could make these therapies a viable therapeutic option for psoriasis patients in the near future.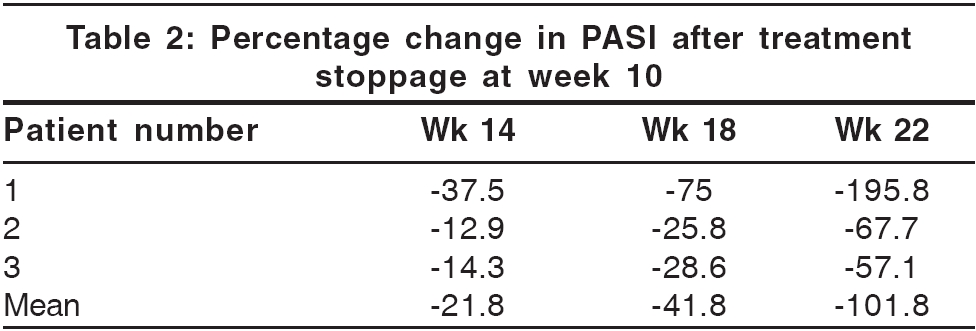


 |
 |
Currently available systemic agents for psoriasis mostly target the proliferating keratinocyte, and their safety, tolerability and need for regular laboratory monitoring are often a concern. The efficacy of cyclosporine and related drugs in psoriasis in the 90s as well as fundamental research over the last decade have improved our understanding of psoriasis as a T-cell mediated disorder.[1],[2]
Though the antigenic triggers that initiate T-cell activation remain to be determined, subsequent steps in the psoriatic T-cell cascade have been well delineated.[1] A number of biologic agents have been designed to target and manipulate these steps. Of these, inhibitors of the cytokine tumor necrosis factor-alpha (TNF-a) have yielded high response rates in patients with psoriasis.[2],[3] Infliximab is a murine-human chimeric monoclonal antibody specific for TNF-a that has been studied extensively in stable plaque psoriasis abroad.[4],[5],[6] In this study, we present our preliminary experience with the use of infliximab in Indian patients with the disease.
Materials and Methods
We conducted an open label pilot study among three patients having moderate to severe plaque psoriasis. All three patients had received methotrexate and PUVA therapy in the past. Additionally, patient 1 had received cyclosporine 1 year ago. Before commencement, a ′washout′ period of 2 weeks for topical therapies and 4 weeks for systemic therapies was observed. Thereafter, baseline PASI assessment as well as hemogram, biochemical profile, ECG, chest radiograph and Mantoux skin tests were performed.
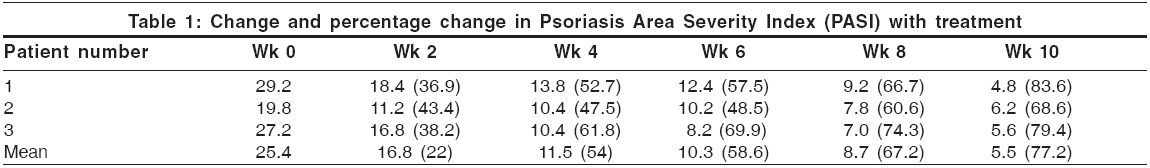
The study had two phases - a 10-week active phase, followed by a 12-week surveillance phase for relapse. During the active phase, infliximab was administered in a dose of 5 mg/kg as an intravenous infusion over 2 h, diluted into 500 ml of normal saline. The dose was repeated at weeks 2 and 6. Monitoring was done at 2-week intervals until week 10 and included PASI, adverse event and laboratory assessments (hemogram, liver and renal function tests). The primary efficacy endpoint was the percentage improvement in PASI at week 10.
During the surveillance phase, PASI assessments were carried out at 4-week intervals until week 22 for relapse. Relapse was defined as loss of 50% or more of the improvement attained in the PASI score at week 10.
Results
Demographic data
The average age was 48.3 years, while the mean duration of psoriasis was 10.3 years.
Effects of treatment
The mean PASI was 25.4 at presentation. At 10 weeks, it declined to 5.5 [Table - 1]. All patients attained PASI 50 (or 50% improvement) by 3.8 weeks. PASI 75 was attained at 9.6 weeks. The mean improvement in PASI at week 10 stood at 77.2%. Relapse occurred at a mean of 18.6 weeks after the first infusion [Table - 2]. Photographs illustrating the typical degree of improvement observed from baseline to week 10 are shown in [Figure - 1]. The first patient, who also had psoriatic arthropathy (PsA), reported partial improvement in spinal stiffness and pain at 2 weeks and peripheral joint stiffness at 6 weeks. These improvements did not diminish at 22 weeks, end of the surveillance period.
Adverse reactions
Infliximab was well tolerated; transient pyrexia and fatigue after infusions were common complaints. Patient 1 had headache and vertigo lasting 5 days after the third infusion, while patient 2 had arthralgia. No laboratory abnormalities were noted.
Discussion
Results from this pilot study indicate that infliximab monotherapy provided significant and rapid benefit to patients with psoriasis, with few adverse effects. Two out of three patients (67%) achieved PASI 75 by week 10, while one patient (Patient 2) showed PASI improvement of 68.6% at week 10, peaking to 70.1% at week 11. In two larger studies.[4],[6] PASI 75 was attained by 87.9% and 88% of patients at week 10. The mean time for onset of clinically meaningful benefit (PASI 50) was 3.8 weeks in this study as compared to 4 weeks in a similar study by Chaudhari et al .[4] Relapse occurred at a mean of 18.6 weeks in this study, as against 26 weeks in a similar study.[5]
Though only minor adverse reactions were encountered in this study, others have documented that infliximab therapy predisposed patients to a higher risk of serious granulomatous and opportunistic infections, particularly reactivation of latent tuberculosis infection (LTBI).[7],[8] This has potentially serious implications in India, where there is a high prevalence of tuberculosis. A thorough history, tuberculin testing and chest radiographs are mandatory during screening. In tuberculin-positive patients, the role of anti-tubercular therapy prior to or concurrently with infliximab administration needs further enquiry.
There is also concern that infliximab could precipitate congestive cardiac failure,[9] hence it is contraindicated in cardiac patients. HACA (Human anti-chimeric antibodies), produced against the murine component of infliximab, may potentially block its action. This has been partly overcome by starting infliximab therapy as a 0-, 2- and 6-week induction regimen and concomitant administration of methotrexate or corticosteroid.[10] Development of lymphoma, demyelinating disease and pancytopenia[9] have been reported with infliximab.
Infliximab therapy currently entails prohibitive costs. An induction regimen of infliximab such as the one used in this study could cost up to Rs. 3,00,000. Maintenance infusions, recommended at 8-week intervals, could cost up to Rs. 10,00,000 per year. With no structured health insurance systems in this country, infliximab is unaffordable for most psoriasis patients. Breakthroughs in biotechnology and large-scale production techniques hold the key to making this therapy more accessible in future.
| 1. |
Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest 2004;113:1664-73.
[Google Scholar]
|
| 2. |
Walsh SR, Shear NH. Psoriasis and the new biologic agents: Interrupting a T-AP dance. Can Med Assoc J 2004;170:1933-41.
[Google Scholar]
|
| 3. |
Tobin AM, Kirby B. TNF-a Inhibitors in the treatment of psoriasis and psoriatic arthritis. BioDrugs 2005;19:47-57.
[Google Scholar]
|
| 4. |
Gottlieb AB, Chaudhari U, Mulcahy LD, Li S, Dooley LT, Baker DG. Infliximab monotherapy provides rapid and sustained benefit for plaque type psoriasis. J Am Acad Dermatol 2003;48:829-35.
[Google Scholar]
|
| 5. |
Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottleib AB. Efficacy and safety of infliximab monotherapy of plaque type psoriasis: A randomised trial. Lancet 2001;357:1842-7.
[Google Scholar]
|
| 6. |
Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: A randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 2004;51:534-42.
[Google Scholar]
|
| 7. |
Gardam MA, Keystone EC, Menzies R, Manners S, Skamene E, Long R, et al. Anti-tumor necrosis factor agents and tuberculosis risk: Mechanisms of action and clinical management. Lancet Infect Dis 2003;3:148-55.
[Google Scholar]
|
| 8. |
Arend SM, Breedveld FC, Van Dissel JT. TNF-a blockade and tuberculosis: Better look before you leap. Neth J Med 2003;61:111-9.
[Google Scholar]
|
| 9. |
Winterfield LS, Menter A. Infliximab. Dermatologic Therapy 2004;17:409-26.
[Google Scholar]
|
| 10. |
Han PD, Cohen RD. Managing immunogenic responses to infliximab: Treatment implications for patients with Crohn's disease. Drugs 2004;64:1767-7.
[Google Scholar]
|
Fulltext Views
2,427
PDF downloads
2,090





