Translate this page into:
Chronic urticaria in patients with autoimmune thyroiditis: Significance of severity of thyroid gland inflammation
2 Department of Internal Medicine, Division of Endocrinology, Ankara, Turkey
3 Department of Biochemistry, Ankara, Turkey
4 Department of Internal Medicine, Division of Allergy, Haydarpasa Training Hospital, Istanbul, Turkey
Correspondence Address:
Ozgur Kartal
GATA Allerjik Hastaliklar BD, Etlik, Ankara
Turkey
| How to cite this article: Gulec M, Kartal O, Caliskaner A Z, Yazici M, Yaman H, Ozturk S, Sener O. Chronic urticaria in patients with autoimmune thyroiditis: Significance of severity of thyroid gland inflammation. Indian J Dermatol Venereol Leprol 2011;77:477-482 |
Abstract
Background: There is a clear association between autoimmune thyroiditis (AT) and chronic urticaria/angioedema (CUA). However, not all patients with AT demonstrate urticaria. Aims: The aim of the study was to investigate in which patients with AT did CUA become a problem. A sensitive inflammation marker, neopterine (NP) was used to confirm whether the severity of inflammation in the thyroid gland was responsible for urticaria or not. Methods: Neopterine levels were assessed in patients with AT with urticaria and without urticaria. Furthermore, levels were compared in relation to pre and post levothyroxine treatment. Twenty-seven patients with urticaria (Group 1) and 28 patients without urticaria (Group 2) were enrolled in the study. A course of levothyroxine treatment was given to all patients, and urine neopterine levels before and after the trial were obtained. Results: All patients completed the trial. Mean age in Group 1 and Group 2 was similar (35.70 ± 10.86 years and 38.36 ± 10.38 years, respectively) (P=0.358). Pre-treatment urine neopterine levels were significantly higher in Group 1 (P=0.012). Post-treatment levels decreased in each group, as expected. However, the decrease in the neopterine level was insignificant in the patients of Group 2 (P=0.282). In Group 1, a significant decrease in post-treatment neopterine levels (P=0.015) was associated with the remission of urticaria. Conclusion: In patients with CUA and AT, pre-treatment elevated levels of NP, and its decrease with levothyroxine treatment along with symptomatic relief in urticaria, may be evidence of the relationship between the degree of inflammation in thyroid and presence of urticaria.Introduction
Chronic urticaria and/or angioedema (CUA) is a common problem and negatively affects both work and social life because of its chronic relapsing course and poor response to therapy. Today, we know that up to 50% of CUA may be autoimmune. [1] There is a wide spectrum of autoimmune disorders associated with CUA. Autoimmune thyroiditis (AT) is one of them. The association between CUA and AT is well established. Several studies on this topic have been published since 1983. [1],[2],[3],[4],[5],[6]
Thyroid hormone replacement therapy, which was first published by Leznoff et al., [2] was found to be effective to improve urticaria, however the underlying immunologic mechanisms of benefit are still unclear. Suppression of thyroid functions with levothyroxine may provide a long-lasting urticaria-free period in many of the patients. [7]
On the other hand, not all patients with AT demonstrate urticaria. In which patients urticaria becomes a problem is still not obvious. One possible explanation is the degree of inflammation. Inflammation in thyroid may be more severe in patients with CUA associated with AT. A marker which positively correlates with inflammation may demonstrate this suggestion.
In this study, we examine the possible correlation between the severity of inflammation in thyroid and clinical findings of urticaria. Neopterine (NP) was used as a marker of inflammation. Neopterine, an acute-phase reactant which can be detectable in body fluids, is a sensitive reflector of monocytes-macrophage activation, and its levels are positively correlated with the severity of inflammation. [8],[9]
Methods
The study was designed as an open study and approved by the local ethical committee. All patients were informed about the study protocol and the effects and side-effects of the drug, and their written consents were obtained.
Study protocol and patient selection: Patients with AT with and without CUA, who were evaluated at the Allergy and Endocrinology outpatient clinics, were included in this prospective study.
Diagnosis of AT was made according to textbook data:
- Elevated thyroid autoantibody levels (anti-thyroid peroxidase and/or anti thyroglobulin)
- Heterogeneous and (other known) parenchymal alterations in thyroid tissue on ultrasonographic examination.
Patients were divided into two subgroups and observed during a study period of 6 weeks (January 2005 to May 2005):
Group 1 consisted of patients with AT and CUA. CUA was defined as recurrent episodes of hives with or without angioedema of at least six-week duration, according to current literature. [10] Etiological factors of CUA other than AT were evaluated by medical history, physical examination and laboratory tests. After a thorough physical examination, laboratory investigations including complete blood count, erythrocyte sedimentation rate, urine and stool microscopy, peripheral blood smear, serum total eosinophil count, serum biochemistry, complement levels, C-reactive protein, rheumatoid factor, HbsAg, anti HCV, antinuclear antibody, anti-double stranded DNA, chest and sinus X-rays and allergy tests with food and inhalant allergens were done. In addition, Helicobacter pylori infection was investigated in those patients with chronic gastric complaints. At the end of the investigations, patients who were positive for any etiological factor that may cause CUA were excluded; the remaining 27 patients with CUA associated with AT were included in the study.
Group 2 consisted of patients with AT without CUA. Patients in this group were also screened for inflammatory or infectious diseases or the use of medicines that could interfere with NP results. Twenty-eight patients were included.
All possible causes for NP elevation were investigated by medical history, physical examination and laboratory tests, e.g. leukocyte count, erythrocyte sedimentation rate, C-reactive protein, and autoantibody screening (other than thyroid auto-ab).
All exclusion criteria are listed below:
- Patients who did not agree to give a written consent
- Patients who had any infection two weeks before the treatment
- Patients who already had any infection
- Patients with diabetes mellitus, coronary heart disease, chronic renal failure, rheumatic diseases
- Patients who underwent a corticosteroid and immunomodulatory therapy during the last month.
Levothyroxine treatment: Levothyroxine, 100 microgram per day was given to all patients in Group 1 and Group 2, for a six-week period. The drug was administered on an empty stomach, 30 min before breakfast to increase gastrointestinal absorption. Until the effective plasma concentration of levothyroxine could be achieved, a short-acting antihistamine (hydroxyzine) was given as a rescue treatment to patients of Group 1. At the beginning of levothyroxine treatment, hydroxizine 25 mg tablet was given two times a day, for seven days, as a rescue treatment. Because the suppressive effect of levothyroxine begins after about a week, when levothyroxine reaches its effective plasma concentration, according to the current literature.
Patients were warned about the importance of daily blood pressure and pulse rate recording during levothyroxine treatment and questioned via phone visits concerning whether an unexpected effect occurred.
Symptom scores in patients with urticaria: The number, duration and proportions of urticaria lesions (plaques), frequency of urticaria attacks and intensity of itching were scored from 0 to 3 (0 = no complaint, 3 = the most bothersome complaints). [11]
Difference between pre and post-treatment urticaria scores was statistically significant (9.74 ± 1.70, and 3.29 ± 1.38, respectively, P<0.001).
Neopterine measurements: Neopterine level was determined in both groups, at baseline and after levothyroxine treatment. A convenient volume of early-morning urine was collected in plastic tubes covered with tin foil. Then, the evaluation samples were stored at -80°C. Urinary NP concentration was measured with an HPLC device (Hewlett Packard 1050; Hewlett Packard, Palo Alto, CA, USA) using a fluorometry detector, as defined by Alrashed et al. [12] A 0.100 mL volume of urine was mixed with 1 mL of mobile phase and injected into a 4 Χ 125 mm C18 reversed phase column; NP was measured by fluorescence from excitation wave length of 353 nm and emission wavelength of 438 nm. Creatinine levels were determined by an ultraviolet detector from absorption at 235 nm. The results were calculated as μmol neopterin/mol creatinine.
Statistical analysis
Statistical analyses were performed by microprocessor using statistical software package (SPSS for windows, SPSS Inc., USA). Mann-Whitney U test and Wilcoxon Signed Ranks test were used to analyze the differences between the groups and to compare pre- and post-treatment results, respectively. All data in the text were expressed as median. A P value <0.05 was accepted as significant.
Study design is summarized in [Figure - 1].
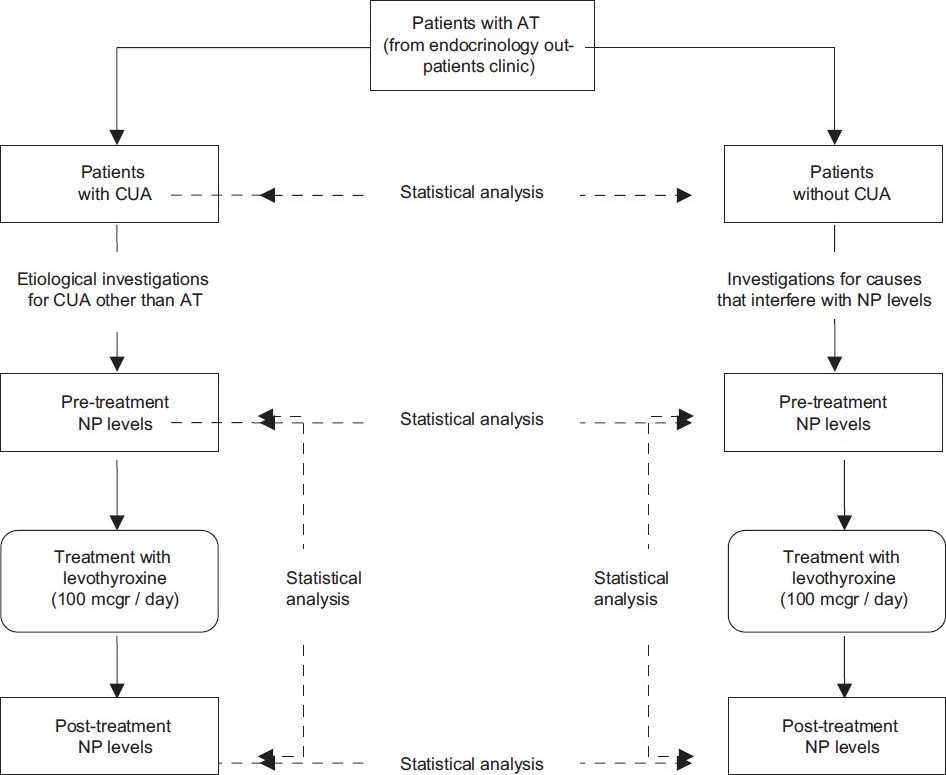 |
| Figure 1: The study design |
Results
Twenty-seven patients (20 females and seven males) in Group 1, 28 patients (18 females and 10 males) in Group 2 completed the trial. The mean age was 35.70± 10.86 years and 38.36± 10.38 years respectively (P=0.358).
Urine NP levels were significantly higher in Group 1 than Group 2 before the levothyroxine treatment (P=0.012) [Figure - 2].
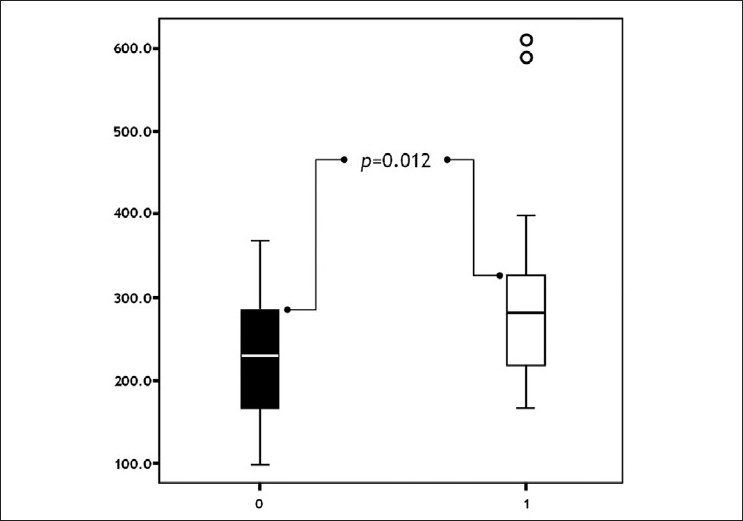 |
| Figure 2: Comparison of groups with respect to pre-treatment neopterine levels ( pts. without urticaria pts. with urticaria) |
Post-treatment NP levels decreased in both groups. The decrease in Group 1 was statistically significant (P=0.015) [Figure - 3]. The reduction in NP levels was parallel to the remission of CUA. Patients benefited from levothyroxine treatment, as expected, as described in the literature at several times. [3],[4],[7] In contrast, the decrease in NP levels was not significant in Group 2, (P=0.282) [Figure - 4].
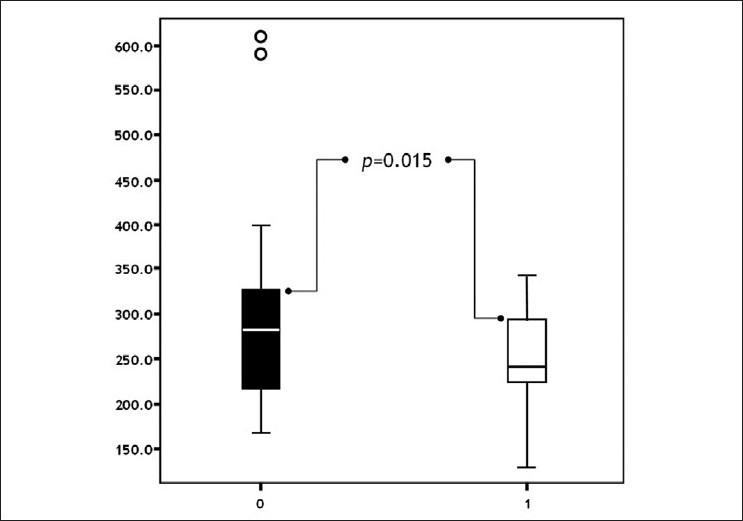 |
| Figure 3: Comparison of pre- and post-treatment neopterine levels in patients with urticaria ( pre-treatment post-treatment) |
 |
| Figure 4: Comparison of pre- and post-treatment neopterine levels in patients without urticaria ( pre-treatment post-treatment) |
The difference of post-treatment NP levels between the groups was insignificant (P=0.091) [Figure - 5]. Results are summarized in [Table - 1].
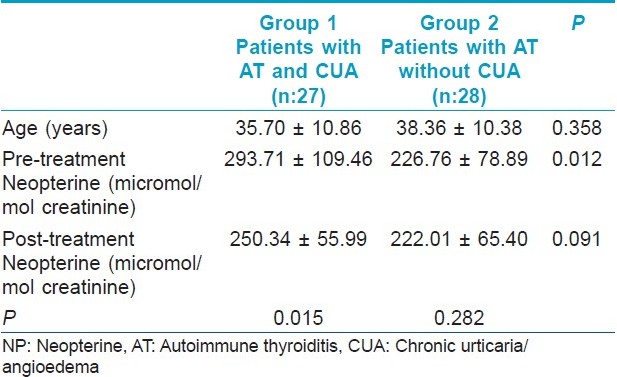
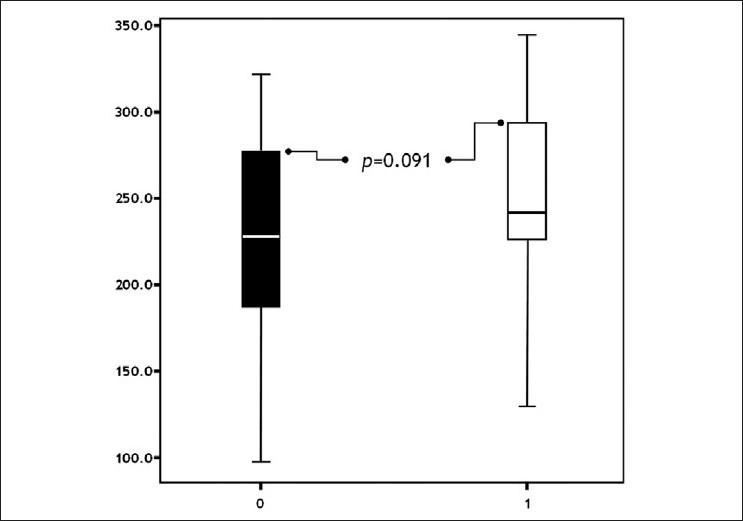 |
| Figure 5: Comparison of groups with respect to post-treatment neopterine levels ( pts. without urticaria pts. with urticaria) |
Discussion
Chronic urticaria is an intensely pruritic, circumscribed, raised, erythematous plaque, often with central pallor and it usually occurs on most days of the week, lasting for a duration of longer than six weeks. It is a common disorder; the lifetime prevalence for any subtype of urticaria is approximately 20%. [10] In many patients with chronic urticaria, no definitive cause can be identified. In such cases, autoimmunity should be mentioned. The presence of mast cell stimulatory serum factors, which cause histamine release, is demonstrated with the autologous serum skin test (ASST), and an autoimmune pathogenic mechanism of chronic urticaria has been suggested. [5],[10],[13],[14]
Chronic autoimmune thyroiditis (Hashimoto′s thyroiditis), is the most common cause of hypothyroidism in iodine-sufficient areas of the world. It is more common in women, and in the age period of 30 to 60. Patients with chronic autoimmune thyroiditis have high serum concentrations of auto antibodies to thyroglobulin (Anti-TG Ab, 50-80%) and thyroid peroxidase (Anti-TPO Ab, 90-100%). [15],[16],[17]
Anti-thyroid antibodies may be positive in patients with chronic urticaria and reversely, urticaria can be seen in patients with Hashimoto′s thyroiditis. It is hypothesized that anti-thyroid antibodies are mast cell-stimulating autoantibodies that bind to either immunoglobulin E (IgE) or to the alpha subunit of the IgE receptor. In this way, the two diseases are associated closely. On the other hand, some studies suggest that chronic urticaria and Hashimoto′s thyroiditis are different clinical presentations of a shared autoimmune process. [18],[19],[20]
Patients with CUA with thyroid autoantibodies may benefit from thyroid hormone replacement therapy. [3],[4],[7],[17],[21] Improvement of urticaria after thyroid hormone replacement may signify the importance of inflammation in the pathogenesis of urticaria. However, not all patients with Hashimoto′s thyroiditis display urticaria. It may be speculated that inflammation in thyroid may be more severe in AT associated with urticaria. A marker that positively correlates with inflammation may demonstrate this suggestion; however, there is no specific marker.
NP, an acute-phase reactant, is a sensitive reflector of monocytes-macrophage activation. NP levels are positively correlated with the severity of inflammation. [22],[23] It is expressed by monocytes-macrophages after interferon-gamma stimulation. Thus, NP was used for diagnosing and following up inflammatory disorders, for example, in HIV infections [24] or in severe acute respiratory syndrome. [25] NP can be measured in both serum and urine, and the results are similar. However, since NP clearances from kidney exist in indestructible form, urine NP levels are directly observed as the produced amounts of NP. [9] Measurement of NP is possible by high-performance liquid chromatography (HPLC), enzyme-linked immunosorbant assays (ELISA) or by radioimmunoassay (RIA).
In our patients with AT and CUA, higher pre-treatment NP levels may be indicative of more severe inflammation in their thyroid glands. After suppression with levothyroxine, NP levels reduced significantly. In addition, although regular antihistamine treatment was not given, CUA symptoms remitted along with the lessening of NP levels. In Group 2 which consisted of patients with AT without CUA, pre-treatment NP levels were low and the post-treatment reduction in levels was insignificant. These results can be interpreted as indicating that the degree of inflammation in the thyroid gland may be a determinant of the presence of urticaria. In other words, urticaria in AT may be directly related to the thyroid gland itself.
Because NP is not a specific marker for thyroid inflammation, an alternative opinion can also be suggested: Could elevated levels of NP be directly related to CUA itself, rather than the thyroid gland? Theoretically, it is possible. However, alteration in NP levels is most likely the consequence of changes in the thyroid gland due to levothyroxine. Most of the previously published studies agree with it, and reveal that healing of CUA symptoms occurs following the suppression of the endogenous thyroid hormones, thyroid-stimulating hormone, and pro-inflammatory mediators by levothyroxine treatment. [4],[7],[26],[27],[28] Indeed, symptomatic relief in urticaria cannot be obtained before levothyroxine achieves its effective plasma concentration. Furthermore, though insignificant, NP levels also decreased in patients with AT without urticaria.
Despite some limitations of the study protocol, our results have signified that patients with AT can experience CUA if the inflammation is severe in their thyroid gland. NP is a sensitive marker, but not specific to thyroid. Studying with a thyroid-specific inflammation marker can yield more accurate results.
| 1. |
Najib U, Sheikh J. The spectrum of chronic urticaria. Allergy Asthma Proc 2009;30:1-10.
[Google Scholar]
|
| 2. |
Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol 1983;119:636-40.
[Google Scholar]
|
| 3. |
Leznoff A, Sussman GL. Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: A study of 90 patients. J Allergy Clin Immunol 1989;84:66-71.
[Google Scholar]
|
| 4. |
Rumbyrt JS, Katz JL, Schocket AL. Resolution of chronic urticaria in patients with thyroid autoimmunity. J Allergy Clin Immunol 1995;96:901-6.
[Google Scholar]
|
| 5. |
Grattan CE, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol 2002;46:645-57.
[Google Scholar]
|
| 6. |
Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol 2005;5:408-12.
[Google Scholar]
|
| 7. |
Karaayvaz M, Caliskaner Z, Turan M, Akar A, Oztürk S, Ozanguc N. Levothyroxine versus ketotifen in the treatment of patients with chronic urticaria and thyroid autoimmunity. J Dermatolog Treat 2002;13:165-72.
[Google Scholar]
|
| 8. |
Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab 2002;3:175-87.
[Google Scholar]
|
| 9. |
Berdowska A, Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. J Clin Pharm Ther 2001;26:319-29.
[Google Scholar]
|
| 10. |
Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, et al. EAACI/GA(2)LEN/EDF/WAO guideline: Definition, classification and diagnosis of urticaria. Allergy 2009;64:1417-26.
[Google Scholar]
|
| 11. |
Breneman D, Bronsky EA, Bruce S, Kalivas JT, Klein GL, Roth HL, et al. Cetirizine and astemizole therapy for chronic idiopathic urticaria: A double-blind, placebo-controlled, comparative trial. J Am Acad Dermatol 1995;33:192-8.
[Google Scholar]
|
| 12. |
Alrashed M, Abougoush M, Akgul EO, Erbil MK. Detection method of serum and urine neopterin levels by high performance liquid chromatography. Gulhane Med J 2002;44:273-80.
[Google Scholar]
|
| 13. |
Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy 2009;39:777-87.
[Google Scholar]
|
| 14. |
Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA(2)LEN task force consensus report: The autologous serum skin test in urticaria. Allergy 2009;64:1256-68.
[Google Scholar]
|
| 15. |
Demirbilek H, Kandemir N, Gonc EN, Ozon A, Alikasifoglu A, Yordam N. Hashimoto's thyroiditis in children and adolescents: A retrospective study on clinical, epidemiological and laboratory properties of the disease. J Pediatr Endocrinol Metab 2007;20:1199-205.
[Google Scholar]
|
| 16. |
Mariotti S, Caturegli P, Piccolo P, Barbesino G, Pinchera A. Antithyroid peroxidase autoantibodies in thyroid diseases. J Clin Endocrinol Metab 1990;71:661-9.
[Google Scholar]
|
| 17. |
Rumbyrt JS, Schocket AL. Chronic urticaria and thyroid disease. Immunol Allergy Clin North Am 2004;24:215-23.
[Google Scholar]
|
| 18. |
Kandeel AA, Zeid M, Helm T, Lillie MA, Donahue E, Ambrus JL. Evaluation of chronic urticaria in patients with Hashimoto thyroiditis. J Clin Immunol 2001;21:335-47.
[Google Scholar]
|
| 19. |
Rottem M. Chronic urticaria and autoimmune thyroid disease: Is there a link? Autoimmun Rev 2003;2:69-72.
[Google Scholar]
|
| 20. |
Zauli D, Deleonardi G, Foderaro S, Grassi A, Bortolotti R, Ballardini G, et al. Thyroid autoimmunity in chronic urticaria. Allergy Asthma Proc 2001;22:93-5.
[Google Scholar]
|
| 21. |
Bangash SA, Bahna SL. Resolution of chronic urticaria and angioedema with thyroxine. Allergy Asthma Proc 2005;26:415-7.
[Google Scholar]
|
| 22. |
Wirleitner B, Reider D, Ebner S, Böck G, Widner B, Jaeger M, et al. Monocyte-derived dendritic cells release neopterin. J Leukoc Biol 2002;72:1148-53.
[Google Scholar]
|
| 23. |
Ray KK, Morrow DA, Sabatine MS, Shui A, Rifai N, Cannon CP, et al. Long-term prognostic value of neopterin: A novel marker of monocyte activation in patients with acute coronary syndrome. Circulation 2007;115:3071-8.
[Google Scholar]
|
| 24. |
Wirleitner B, Schroecksnadel K, Winkler C, Fuchs D. Neopterin in HIV-1 infection. Mol Immunol 2005;42:183-94.
[Google Scholar]
|
| 25. |
Zheng B, Cao KY, Chan CP, Choi JW, Leung W, Leung M, et al. Serum neopterin for early assessment of severity of severe acute respiratory syndrome. Clin Immunol 2005;116:18-26.
[Google Scholar]
|
| 26. |
Aversano M, Caiazzo P, Iorio G, Ponticiello L, Laganá B, Leccese F. Improvement of chronic idiopathic urticaria with L-thyroxine: A new TSH role in immune response? Allergy 2005;60:489-93.
[Google Scholar]
|
| 27. |
Gaig P, García-Ortega P, Enrique E, Richart C. Successful treatment of chronic idiopathic urticaria associated with thyroid autoimmunity. J Investig Allergol Clin Immunol 2000;10:342-5.
[Google Scholar]
|
| 28. |
Zauli D, Grassi A, Ballardini G, Contestabile S, Zucchini S, Bianchi FB. Thyroid autoimmunity in chronic idiopathic urticaria: Implications for therapy. Am J Clin Dermatol 2002;3:525-8.
[Google Scholar]
|
Fulltext Views
5,297
PDF downloads
2,471





