Clinical efficacy and safety of upadacitinib in the treatment of palmoplantar pustulosis: A single-center retrospective study
Corresponding authors: Dr. Suju Luo, Department of Dermatology, Tianjin Medical University General Hospital, 154 Anshan Dao, Tianjin, China. luosuju@tmu.edu.cn
Runping Yang, Department of Dermatology, The Sixth Medical Center of Chinese PLA General Hospital, Beijing, China. yrp809@163.com
-
Received: ,
Accepted: ,
How to cite this article: Zheng Y, Zhang X, Wang H, Yang R, Luo S. Clinical efficacy and safety of upadacitinib in the treatment of palmoplantar pustulosis: A single-center retrospective study. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_751_2024
Dear Editor,
Palmoplantar pustulosis (PPP) is a rare, chronic, recurrent disease characterised by sterile pustules on the palms and soles. The prevalence of PPP is approximately 0.01– 0.05%. 1 Janus kinase (JAK) inhibitors, including upadacitinib, have been clinically used to treat PPP.2 In this study, we retrospectively analysed the data of 20 PPP patients who received upadacitinib treatment and evaluated its therapeutic efficacy and safety. These patients had previously undergone systemic treatment and/or topical therapy but showed poor response. A chest computed tomography (CT) scan was performed before treatment to detect active tuberculosis. We excluded patients with active tuberculosis, viral infections, severe liver and kidney disease, cardiovascular conditions, malignant diseases, or those who could not comply with treatment recommendations and follow-up requirements. Upadacitinib was administered orally at doses of 15, 30, or 45 mg daily. During the follow-up period (median: 12 weeks; range: 0 to 24 weeks), routine blood tests were performed, and any adverse reactions were recorded. Palmoplantar Pustulosis Area and Severity Index (PPPASI) and Physician Global Assessment (PGA) scores before and after treatment were compared. A 50%, 75%, and 90% improvement in PPPASI was referred to as PPPASI 50, PPPASI 75, and PPPASI 90, respectively. Quantitative data were compared using Student’s t-tests and P < 0.05 was considered significant.
The PPP patients included in this study comprised 6 males and 14 females, with a mean age of 49.50 ± 10.96 years. The patients’ characteristics are detailed in Table 1. Some patients had previously undergone systemic therapies with adalimumab (a monoclonal antibody against tumor necrosis factor α (TNF-α)), ixekizumab (a humanised monoclonal antibody against interleukin-17 (IL-17)), secukinumab (a monoclonal antibody against IL-17), acitretin, and traditional Chinese medicine. The previous treatments were discontinued due to inadequate response. Upadacitinib was used as a rescue treatment for some patients with PPP who showed inadequate response to conventional therapies and biologics. Of the 20 PPP patients, 16 received 15 mg/day of upadacitinib, 3 received 30 mg/day, and 1 received 45 mg/day [Table 2]. After 12 weeks of upadacitinib treatment, erythema, pustules, and desquamation on the palms and soles subsided, as shown in Figures 1a-f and Figure 2a-d. The average PPPASI score decreased significantly from 17.69 ± 11.29 to 1.27 ± 1.41, with the proportions achieving PPPASI 50, PPPASI 75, PPPASI 90, and PPPASI 100 being 100%, 100%, 50%, and 20%, respectively (P < 0.05); [Supplementary Table S1]. The PGA score also significantly improved from 2.70 ± 0.90 to 0.45 ± 0.60 (P < 0.05); [Supplementary Table S1]. No serious adverse events occurred during the treatment period.
| Case. | Gender | Age (years) |
Disease duration |
Joint pain | Nail disease | Comorbid conditions | Family history | PsO | AD | Previous treatments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 59 | 1 month | Sacroiliac and ankle joints | N | Hyperlipidemia, urticaria | N | N | N |
Tripterygium glycosides 20 mg, tid, for six months caused liver function abnormalities, Acitretin capsules 20 mg/d, ebastine |
| 2 | F | 38 | 3 months | Spinaljoint, right sternoclavicular joint | N | N | N | N | N | TCM, Topical medicine (complex formulation of phellodendron, clobetasol, beclomethasone, tazarotene) The effect was not significant. |
| 3 | M | 60 | 10 years | N | Y | N | Y | Y | N | Adalimumab was administered at 80 mg initially, followed by 40 mg every 2 weeks after one week, with no significant improvement in lesions after 5 treatments AD-like rashes appeared on the limbs, with severe itching. |
| 4 | F | 32 | 6 years | N | N | N | N | Y | N | Ixekizumab was administered at 160 mg at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks for a total of 2 years, after which the disease relapsed. |
| 5 | F | 40 | 1 year | N | Y | N | N | N | N |
TCM The effect was not significant. |
| 6 | M | 37 | 2 months | Sterno-clavicular joint | Y | Hyperglycemia | N | N | N |
TPSO The effect was not significant. |
| 7 | F | 55 | 4 month | N | Y | N | N | N | N |
TPSO, Terbinafine, Terbinafine cream The effect was not significant. |
| 8 | M | 44 | 8 months | N | N | N | Y | Y | N |
TPSO The effect was not significant. |
| 9 | M | 54 | 2 months | N | Y | Diabetes | N | N | Y |
TCM, TPSO The effect was not significant. |
| 10 | M | 45 | 3 months | N | N | N | N | N | N |
Clobetasol/triclosan cream The effect was not significant. |
| 11 | F | 48 | 10 years | Sternoclavicular, bilateral shoulder, bilateral hands distal interphalangeal joints | Y | N | N | Y | N | After seven treatments with secukinumab, there was no significant improvement. Treatment was switched to adalimumab, and after two treatments, the rashes on the trunk and limbs, as well as joint pain, were alleviated compared to before. However, new pustules still appeared on the hands and feet. Methotrexate was administered orally at 10 mg per week, resulting in the gradual resolution of the pustules on the hands and feet, with no significant joint pain. After 24 weeks of treatment, AD-like rashes appeared on the lower legs, with severe itching affecting daily life. |
| 12 | F | 58 | 9 months | Left knee, cervical spine | Y | Uveitis | N | N | Y |
TPSO The effect was not significant. |
| 13 | F | 55 | 2 months | Sternoclavicular, bilateral knees, hips, shoulders, elbows, bilateral hands interphalangeal joints | N | Rhinitis, pharyngitis | N | N | N | Adalimumab was administered at 80 mg initially, followed by 40 mg every 2 weeks after one week, with no significant improvement in lesions after 12 weeks AD-like rashes appeared on the limbs, with severe itching, Leflunomide 10 mg qd for 3 months, Clobetasol/triclosan cream, Calcipotriol ointment. |
| 14 | F | 33 | 10 years | N | N | Hypothyroidism | N | N | Y |
TCM, TPSO The effect was not significant. |
| 15 | M | 44 | 2 years | Sterno-clavicular, hip, left foot first metatarsophalangeal joint | Y | N | Y | Y | N | Adalimumab, Thalidomide |
| 16 | F | 46 | 7 years | N | Y | Hyperlipidemia, gallstones | N | N | N |
TCM, TPSO The effect was not significant. |
| 17 | F | 59 | 7 months | Sternum | Y | Hypertension, diabetes, angina | N | N | N | Tripterygium glycosides, Sulfasalazine, Clobetasol/triclosan cream The effect was not significant. |
| 18 | F | 53 | 3 weeks | Shoulder | N | Ulcerative colitis | N | N | N | Untreated |
| 19 | F | 77 | 2 months | Right ankle | Y | Hyperglycemia, hyperlipidemia | N | N | N | N |
| 20 | F | 57 | 3 months | N | Y | Hypertension, hypothyroidism | N | N | N | Acitretin 20 mg orally for 3 months resulted in elevated blood lipids. |
F: female; M: male; PsO: Psoriasis; AD: Atopic Dermatitis; N: none; Y: yes; TCM: Traditional Chinese medicine; TPSO: Topical potent steroid ointment.
| Case. | Upadacitinib Dose | Systemic Treatment Concomitance | Topical Treatment Concomitance | PGA Before Treatment | PGA at 12 Weeks | PPPASI Before Treatment | PPPASI at 12 Weeks | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| 1 | 15 mg | Ebastine | N | 2 | 0 | 10.8 | 0 | N |
| 2 | 15 mg | N | Calcipotriol, Clobetasol/triclosan | 2 | 0 | 6.2 | 0 | N |
| 3 | 30 mg | N | N | 5 | 1 | 48.6 | 3.8 | N |
| 4 | 15 mg | N | N | 2 | 0 | 6.4 | 0 | N |
| 5 | 15 mg | N | N | 2 | 0 | 5.4 | 0 | N |
| 6 | 15 mg | Ebastine | Calcipotriol, Clobetasol/triclosan | 4 | 1 | 22.2 | 1.1 | N |
| 7 | 15 mg | N | Triamcinolone acetonide/urea cream | 2 | 0 | 11.2 | 0 | N |
| 8 | 15 mg | N | N | 3 | 0 | 11 | 0 | N |
| 9 | 15 mg | N | N | 2 | 0 | 10.8 | 1.2 | N |
| 10 | 15 mg | N | N | 2 | 0 | 8.8 | 1.1 | N |
| 11 | 30 mg | Ebastine | N | 2 | 1 | 12 | 2 | N |
| 12 | 15 mg | N | N | 2 | 0 | 6.2 | 0 | N |
| 13 | 30 mg | N | Calcipotriol, Clobetasol/triclosan | 3 | 1 | 24 | 2.2 | N |
| 14 | 15 mg | N | N | 2 | 0 | 8.4 | 1.2 | N |
| 15 | 15 mg | N | N | 4 | 2 | 24 | 2.6 | N |
| 16 | 15 mg | N | N | 4 | 2 | 32 | 5.2 | N |
| 17 | 15 mg | N | N | 3 | 0 | 25.8 | 1.2 | N |
| 18 | 45 mg | N | N | 2 | 0 | 21 | 0 | Transient increase in blood pressure |
| 19 | 15 mg | N | N | 3 | 0 | 27 | 1.2 | N |
| 20 | 15 mg | N | Calcipotriol, Triamcinolone acetonide | 3 | 1 | 32 | 2.6 | N |
N: none; PPPASI: Palmoplantar Pustulosis Area and Severity Index; PGA: Physician Global Assessment
The PPPASI improvement rate was calculated as follows: PPPASI Score = Right palm [(Erythema + Pustules + Desquamation) × Area ×0.2]+Left palm [(Erythema + Pustules + Desquamation) × Area × 0.2] + Right sole [(Erythema + Pustules + Desquamation) × Area × 0.3] + Left sole [(Erythema + Pustules + Desquamation) × Area × 0.3]. Erythema, pustules, and desquamation scores: 0 = absent, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe.
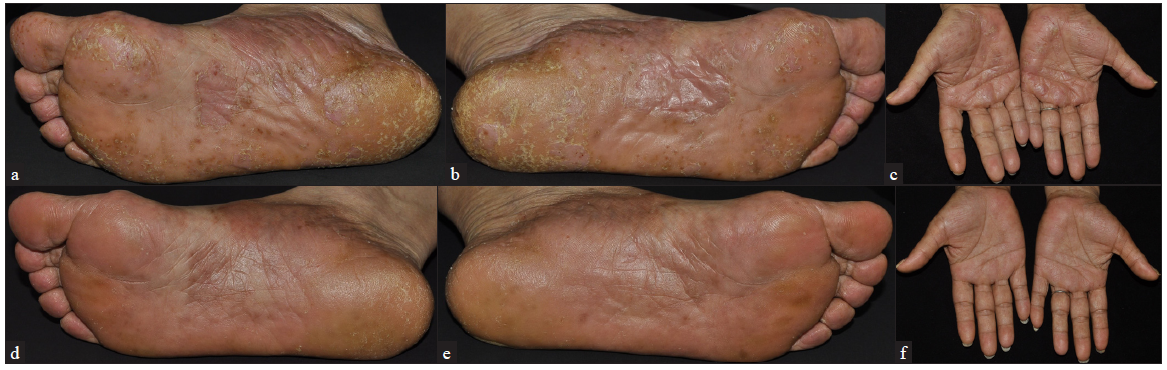
- A 59-years old woman with palmo-plantar lesions at baseline (a-c) and after 12-weeks of upadacitinib treatment (d-f).
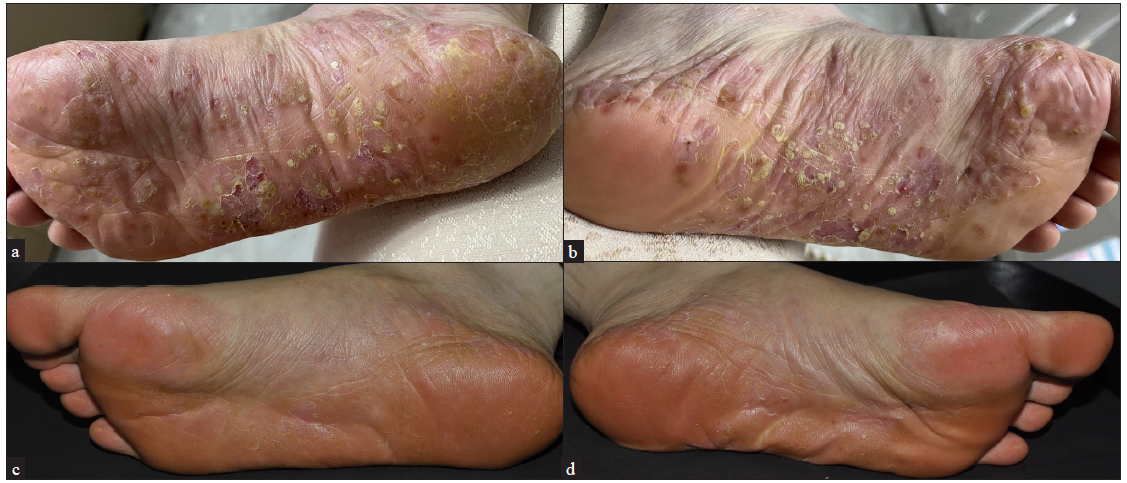
- A 38-year-old women in 8 months postpartum with PPP, before Upadacitinib treatment (a-b) and after 12 weeks of updacitinib treatment (c-d).
Upadacitinib is currently approved for various pathological conditions, including atopic dermatitis (AD), psoriatic arthritis (PsA), rheumatoid arthritis (RA), and ulcerative colitis (UC).3 It can be utilised to treat AD-like rashes that occur during biological treatment for PsA. In this study, two patients with PPP and PsA developed AD-like rashes following injections of adalimumab and secukinumab. They then switched to upadacitinib treatment, which resolved AD-like rashes. It has been documented that 5–20% of patients receiving TNF-α antagonists for various inflammatory diseases develop AD-like rashes.4 Disruption of the balance between pro- and anti-inflammatory cytokines may account for the development of AD-like rashes after treatment with TNF-α antagonists. Upadacitinib can also be used to treat PPP manifestations that occur following the treatment of immune diseases such as UC and RA. One of our patients, who had a 10-year history of UC, developed PPP manifestations after treatment with vedolizumab (a monoclonal antibody against α4β7 integrin) and infliximab (a monoclonal antibody against TNF-α). According to the instructions for use, the patient received oral upadacitinib (45 mg/day), resulting in a marked improvement in joint pain and rashes. During the treatment period, no adverse effects were observed except for a transient increase in blood pressure. It has been reported that oral administration of upadacitinib (15 mg/day) effectively mitigates skin lesions in two PPP patients with PsA who exhibited a poor response after the IL-17 inhibitor treatment.5 Similar findings were observed in our cohort of 10 PPP patients with PsA, confirming the therapeutic value of upadacitinib for PPP patients.
A retrospective study design, a small sample size, and a short follow-up period are the main limitations of this study. Potential adverse events associated with the use of JAK inhibitors include cardiovascular diseases, thrombosis, and infections.6 Therefore, during the treatment with upadacitinib, routine blood and coagulation function tests are necessary.
In conclusion, upadacitinib is effective in relieving the symptoms of PPP. However, the long-term safety of upadacitinib still requires further investigation.
Ethical approval
The research/study was approved by the Institutional Review Board at Tianjin Medical University General Hospital, number IRB2024-YX-006-01, dated 2023-01.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
National Natural Science Foundation of China, Grant/Award Number: 81773319; Tianjin Key Medical Discipline (Specialty) Construction Projectuff08No:TJYXZDXK-057Buff09
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Recent advances in palmoplantar pustulosis. Fac Rev. 2021;10:62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Palmoplantar pustulosis successfully treated with upadacitinib: Report of three cases. Australas J Dermatol. 2023;64:568-70.
- [CrossRef] [PubMed] [Google Scholar]
- The JAK-STAT pathway at 30: Much learned, much more to do. Cell. 2022;185:3857-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Eczema as an adverse effect of anti-TNFα therapy in psoriasis and other Th1-mediated diseases: A review. Dermatol Treat. 2017;28:237-241.
- [Google Scholar]
- Palmoplantar pustulosis with psoriatic arthritis ineffective to interleukin-17 inhibitors: two patients successfully treated with upadacitinib. J Dermatology Treat. 2023;34:2280508.
- [CrossRef] [Google Scholar]
- English version of Japanese guidance for the use of oral Janus kinase inhibitors (JAK1 and TYK2 inhibitors) in the treatments of psoriasis. J Dermatol. 2023;50:e138-e150.
- [CrossRef] [PubMed] [Google Scholar]






