Translate this page into:
Clinico-epidemiological characteristics and long-term surgical outcome of basal cell carcinoma treated with standard excision in patients of skin of colour: A retrospective study from Northern India
Corresponding author: Dr. Sunil Dogra, Department of Dermatology, Venereology and Leprology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. sundogra@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vinay K, Mehta H, Chatterjee D, Reddy A, Jain S, Narang T, et al. Clinico-epidemiological characteristics and long-term surgical outcome of basal cell carcinoma treated with standard excision in patients of skin of colour: A retrospective study from Northern India. Indian J Dermatol Venereol Leprol. 2025;91:210-6. doi: 10.25259/IJDVL_717_2023
Abstract
Background
There is scant data on basal cell carcinoma (BCC) in Indian patients. This retrospective study was conducted to explore epidemiology, risk factors, clinical and pathological aspects, and long-term treatment outcomes of BCC in a cohort of North Indian patients.
Methods
Data about patients registered in the dermatosurgery clinic between 01 January 2017 and 31 December 2022 with a confirmed diagnosis of BCC was collected.
Results
Among the 83 patients, 56.6% were females, and the median age was 62 years (6–85 years). Most patients (81.9%) had a single BCC lesion, resulting in a total of 126 assessed lesions. The median size of BCC at presentation was 1.90 cm, with nodular BCC being the most common histopathological subtype (39.7%). Head and neck region involvement was observed in 82.5% of patients, with the malar region, nose, and periorbital region being the most commonly affected sites. Pigmentation was clinically evident in 45.2% of cases. Surgical excision was the primary treatment modality (71.1% of patients). The median follow-up duration was 40 months (6–57 months). Recurrence occurred in five patients, with a longer disease-free survival period observed in the surgically treated group (55.58 ± 0.98 months) compared to patients treated with medical or destructive therapies (43.6 ± 3.482 months) (p = 0.003).
Conclusion
The data from this hospital-based study indicated a slight predilection for females among North Indian patients with BCC, with most cases occurring during their seventh decade of life. The condition commonly occurred on sun-exposed areas such as the malar region and nose, with a high percentage of pigmented lesions. Recurrence following surgical excision was rare, and overall treatment outcomes were favourable.
Keywords
Basal cell carcinoma (BCC)
skin of colour
treatment outcomes
surgical excision, Mohs micrographic surgery
Introduction
Basal cell carcinoma (BCC) is the most common cutaneous malignancy worldwide, accounting for nearly 80% of all non-melanoma skin cancers.1 While they have low metastatic potential, BCCs are locally invasive. Risk factors include radiation exposure, human papillomavirus infections, immunosuppression, and genetic predisposition.2 BCCs are more common in older individuals and often affect men more than women.3
A skin biopsy is essential to confirm the diagnosis, although dermoscopy can aid in clinical diagnosis.4 Most primary BCCs are managed with surgical excision. Destructive measures and topical treatments are alternatives for low-risk patients or when surgery is impractical.5 Prognosis is excellent with low recurrence rates, but regular follow-up is vital. Around 50% of recurrences appear within 2 years and 80% within 5 years post-treatment.6
Incidence rates of BCC are rising in Caucasians and Asians alike.7–9 Aggressive subtypes are rising, highlighting the need for early diagnosis.10 Most BCC literature concentrates on phototypes I and II, which are common in Caucasians. However, BCC in the skin of colour often presents differently, being pigmented and prone to misdiagnosis, which requires a high index of suspicion among clinicians.11
While prior Indian studies have covered BCC demographics and clinical features, data on long-term follow-up and treatment outcomes are lacking.12–15 This retrospective study aimed to investigate the epidemiology, risk factors, clinical and pathological aspects, and treatment outcomes of BCC in North Indian patients.
Methods
This retrospective study, conducted at a North Indian tertiary care centre, focused on patients diagnosed with BCC at the dermatosurgery clinic from January 1, 2017, to December 31, 2022. Inclusion criteria comprised a confirmed clinical and histopathological diagnosis of BCC, along with the availability of detailed clinical history, dermoscopic features, and histopathology findings. Demographic parameters (age, gender, Fitzpatrick skin type) and potential risk factors (chemical exposures, genodermatoses, smoking, personal/family history of skin cancers, use of photoprotective measures, comorbidities, and prior treatments) were systematically collected.
Clinical examination recorded details about BCC lesions, including location, size, number, and morphology. Treatment modalities, such as standard surgical excision, Mohs micrographic surgery, topical agents (5-fluorouracil, imiquimod), curettage, and radiofrequency ablation, were chosen based on clinical features, risk assessment, and patient preferences. Surgical options were provided for patients fit for surgery, with Mohs micrographic surgery preferred in sensitive areas or cases with aggressive subtypes or recurrence. Patients with multiple lesions or compromised health were directed toward topical or locally destructive therapies.
We adhered to evidence-based guidelines for BCC management. Histopathological and dermoscopic findings were extracted from records. Only participants with complete details were included, excluding those with missing data. For follow-up, we specifically considered patients with a minimum follow-up duration of 6 months. Patients were informed about potential BCC recurrence and encouraged to report any symptoms promptly, even if they occurred between scheduled follow-up visits conducted every 6 months.
We employed SPSS version 23 for data analysis, expressing qualitative data as percentages and quantitative data as medians and ranges. Pairwise comparisons utilised Chi-square or Fisher’s exact test for qualitative data and Mann–Whitney U test for skewed quantitative data. Analyses were two-sided with p = 0.05 significance. Survival analysis used Kaplan–Meier curves and the Cox proportional hazards regression model assessed predictor variables’ impact on recurrence risk.
Results
Population demographics and risk factors
Out of 83 confirmed cases, 47 (56.6%) were females. The median age was 62 years (6–85). Nearly one-third were in their seventh decade of life. Median age at presentation showed no statistically significant gender difference (p = 0.865). Mean (SD) symptom duration was 46.6 (51.3) months (1–360) and was similar for males and females (p = 1.00). Excluding the patient with oculocutaneous albinism, all patients were Fitzpatrick skin types III, IV, or V. Table 1 presents an overview of the demographic characteristics of the study population, encompassing associated genodermatoses, comorbidities, and diverse risk factors.
| Characteristics | Value |
|---|---|
| Total confirmed cases | 83 |
| Females (%) | 47 (56.6%) |
| Median age in years (Range) | 62 (6–85) |
| Mean (SD) symptom duration in months (Range) | 46.6 (51.3) (1–360) |
| Genodermatoses (%) | 8 (9.6%) |
| - Xeroderma pigmentosum | 5 |
| - Gorlin syndrome | 2 |
| - Albinism | 1 |
| - Epidermodysplasia verruciformis | 1 |
| Common comorbidities | |
| - Hypertension | 17 |
| - Diabetes | 16 |
| Prior malignancies | |
| - Squamous cell carcinomas | 2 |
| - Laryngeal carcinoma | 1 |
| - Hepatocellular carcinoma | 1 |
| Concurrent premalignant lesions | |
| - Bowen’s disease | 2 |
| - Actinic keratosis | 1 |
| Fitzpatrick skin types | |
| - III, IV or V | All patients (excluding albinism) |
| Photoprotective measures (%) | 8 (9.6%) |
| Risk factors | |
| - Smoking | 6 (7.2%) |
| - Family history of BCC | 3 (3.6%) |
| - Exposure to ionising radiation | 2 (2.4%) |
| - Iatrogenic immunosuppression | 1 (1.2%) |
| Regular daily outdoor activities (%) | Around 70% |
SD: Standard deviation
Clinical, dermoscopic, and histopathologic characteristics
The majority (81.9%) had a single lesion, while 15 had multiple lesions, including six with genodermatoses and one who was a liver transplant recipient on immunosuppressants. Table 2 summarises the clinical details of the study population. In total, 126 lesions were assessed. The median size at presentation was 1.90 cm (0.2 cm–7 cm). Patients with lesions exceeding 2 cm had a significantly longer median duration of illness (48.0 (60.0) vs 24.0 (49.5) months, p = 0.048). Among 126 examined lesions, 104 (82.5%) were present on the head and neck. The most common site of involvement was the malar region, with right and left cheeks [Table 3]. Pigmentation was clinically evident in 57/126 patients (45.2%). The most common dermoscopic features observed were crusting (n = 71, 56.3%), arborising telangiectasias (n = 69, 54.7%), and blue-grey dots (n = 65, 51.5%) [Table 4].11 Of the 88 lesions for which clinico-histopathological typing could be done, the most common subtype was nodular BCC in 35 patients (39.7%). Other observed clinico-histopathological variants included nodulocystic (n = 21, 23.8%), micronodular (n = 17, 19.3%), superficial (n = 7, 7.95%), basosquamous (n = 6, 6.8%), morpheaform BCC (n = 1, 1.14%) and infiltrative BCC (n = 1, 1.14%).
| Lesion Characteristics | Details |
|---|---|
| Number of lesions | 126 |
| Clinically evident pigmentation | 57/126 patients (45.2%) |
| Lesion margins | Rolled: 72 (57.1%), raised: 48 (38.1%) and depressed: 6 (4.7%) |
| Lesion surface | Ulcerated: 46 (36.5%), crusted: 44 (34.9%), smooth: 14 (11.1%), telangiectatic: 9 (7.1%), verrucous: 7 (5.5%) and gritty: 6 (4.8%) |
| Lesion size (Median) | 1.90 cm (Interquartile range (IQR): 1.82 cm) |
| Lesion size range | 0.2 cm–7 cm |
| Symptoms | Asymptomatic: 58 patients (69.9%), bleeding: 21 patients (25.3%) and pain: 8 patients (9.6%) |
| Recurrent lesions | 5 patients (3.97%) |
| Treatment of recurrent lesions | Surgical treatment: 2 patients, electrosurgery: 1 patient, topical 5-Fluorouracil: 1 patient, radiotherapy: 1 patient |
| Duration of illness (Median) | Exceeding 2 cm: 48.0 (60.0) months, not exceeding 2 cm: 24.0 (49.5) months, p = 0.048 |
| Site | Number of lesions (n = 126) |
|---|---|
| Malar region | 33 (26.1%) |
| Nose | 26 (20.6%) |
| Periocular | 11 (8.7%) |
| Periauricular | 10 (7.9%) |
| Forehead | 7 (5.5%) |
| Temple | 5 (3.9%) |
| Chin | 5 (3.9%) |
| Neck | 5 (3.9%) |
| Scalp | 2 (1.6%) |
| Lip | 2 (1.6%) |
| Other (trunk and limbs) | 20 (15.8%) |
| Dermoscopic changes | Number of lesions (n = 126) | |
|---|---|---|
| Surface changes | Scaling/crusting | 71 (56.3%) |
| Ulceration | 51 (40.4%) | |
| Erosions | 38 (30.1%) | |
| Pigmentary changes | Blue-grey dots | 65 (51.5%) |
| Blue-white veil | 62 (49.2%) | |
| Maple leaf-like areas | 59 (46.8%) | |
| Blue-grey globules | 53 (42.1%) | |
| Spoke wheel areas | 8 (6.3%) | |
| Regression structures | Yellow-white structureless areas | 31 (24.6%) |
| Short white streaks | 15 (11.9%) | |
| Vascular changes | Arborising vessels | 69 (54.7%) |
| Linear telangiectasias | 41 (32.5%) | |
| Hairpin vessels | 10 (7.9%) | |
| Glomerular vessels | 14 (11.1%) | |
| Special signs | Adherent fibre sign | 27 (21.4%) |
| Rainbow effect | 34 (26.9%) | |
Management, complications, and long-term follow-up
Modes of management included surgical excision in 59 patients (71.1%, [Figure 1]) and Mohs micrographic surgery in four patients (4.8%, [Figure 2]). Six patients received topical therapy, including 5-fluorouracil (n = 5, 7.2%) and imiquimod (n = 1,1.2%). Radiofrequency ablation and curettage were employed in two patients each (2.4%). Three patients denied intervention, and seven patients were referred to the oculoplastic/plastic surgery department for management. Surgical excision was performed with a margin of 3–4 mm and reconstruction was performed by various methods including primary closure (n = 26, 44.1%), advancement flap (n = 15, 25.4% [Figures 1 and 2]), rotation flap (n = 8, 13.5%, [Figures 3 and 4]), transposition flap (n = 6, 10.2%, [Figure 5]) and bilobed flap (n = 4, 6.7%). Short-term complications included local site infection (n = 8), wound gaping (n = 6) and flap necrosis (n = 2). Over the long term, two patients developed hypertrophic scarring.
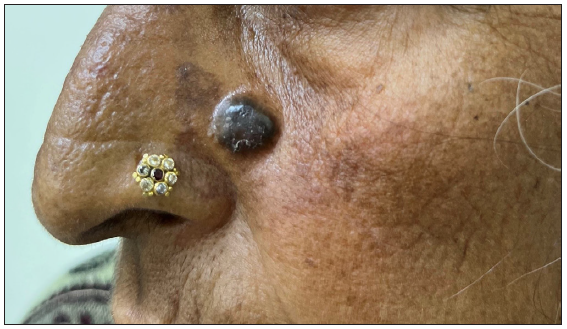
- A case of nodular pigmented basal cell carcinoma over the lateral wall of the nose.
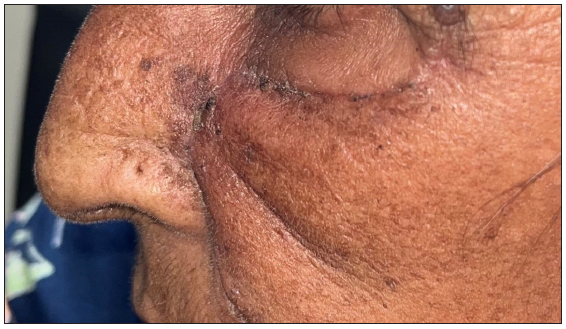
- Post-operative clinical photographs after standard surgical excision and advancement flap.
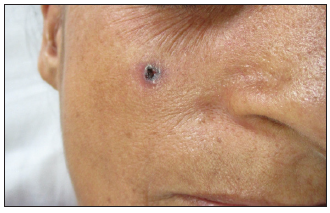
- A clinical case illustrating the management of basal cell carcinoma on the cheek using surgical excision followed by advancement flap.
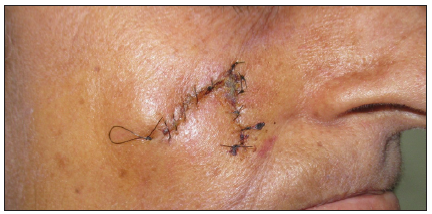
- Post-operative image taken at 7 days after the surgical procedure.
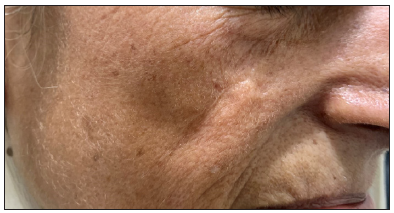
- A photograph captured 8 weeks post-surgery, demonstrating a well-healed but slightly depressed scar.
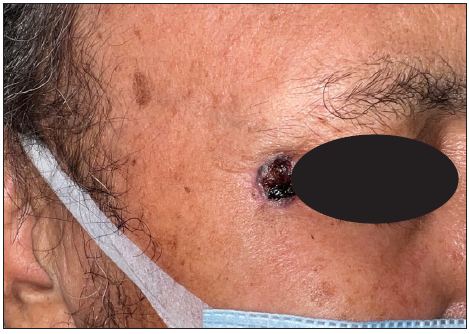
- A case of micro-nodular basal cell carcinoma over the lateral margin of the eye showing an ulcerative surface and beaded margin.
- Post-treatment photographs after Mohs micrographic surgery and rotational flap.
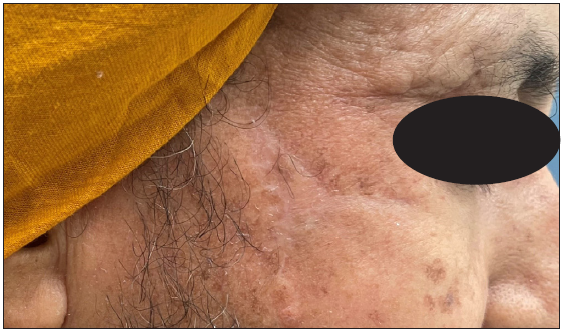
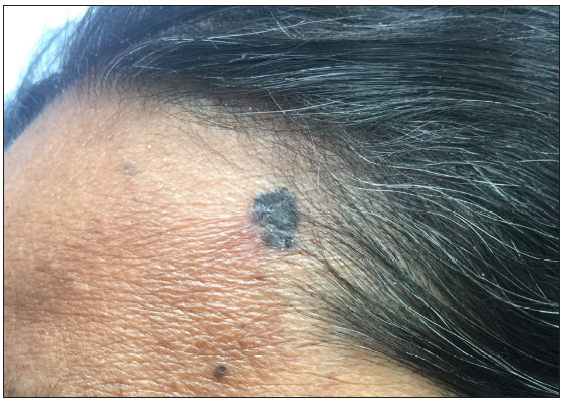
- Clinical presentation of basal cell carcinoma on the medial cheek of an elderly woman.
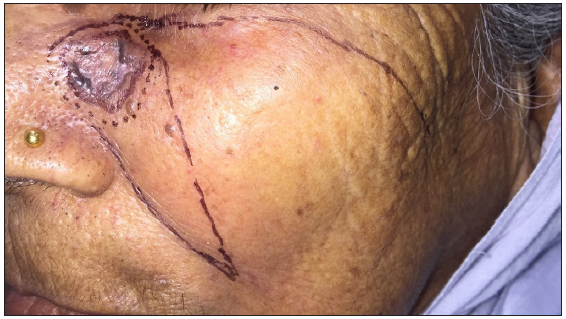
- Pre-operative surgical markings delineating excision margins and flap reconstruction plans.
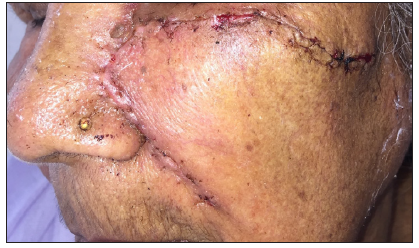
- Post-operative images captured at approximately 7 days post-surgery, revealing a healthy suture line.
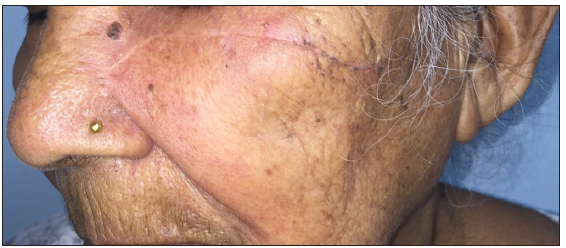
- Follow-up images obtained after 8 weeks, demonstrating a well-healed, thin, linear scar.
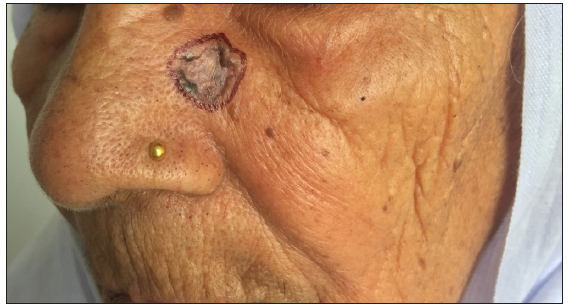
- A case of basal cell carcinoma over the scalp margin.
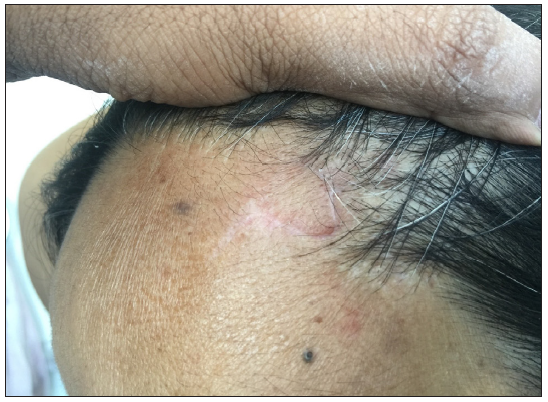
- Post-operative photographs after surgical excision and rhomboid flap.
Of the 70 patients with available follow-up (median: 40 months, range: 6–57 months), five (7.1%) had recurrences, comprising two surgical excision patients, two treated with 5-fluorouracil, and one with curettage [Table 5]. An additional 7.1% developed new BCCs at different sites, which included two who also had xeroderma pigmentosum. Three patients died, one from lung cancer, another from erythroderma secondary to pityriasis rubra pilaris, and the third from multiorgan failure.
| S. no. | Age in years/gender | Duration of disease at presentation in months | Risk factor(s) | Site of lesion | Size of lesion (in cm) | Clinico-histological subtype | Initial management | Time to recurrence (in months) | Management of recurrent lesion |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 21/M | 7 | Xeroderma pigmentosum, recurrent lesion | Temple | 0.5 × 0.5 | Basosquamous | Surgical excision with primary closure | 46 | Re-excision |
| 2. | 47/F | 48 | None | Chin | 3×2 | Micronodular | Surgical excision with advancement flap | 40 | Denied intervention |
| 3. | 47/F | 12 | Epidermodysplasia verruciformis | Nose | 4×3 | Superficial | Topical 5-fluorouracil | 26 | Denied intervention |
| 4. | 65/M | 36 | Photo damage, including multiple actinic keratoses | Pre-auricular | 5×2 | Nodulocystic | Topical 5-fluorouracil | 32 | Surgical excision |
| 5. | 6/M | 2 | Xeroderma pigmentosum | Malar | 1×1 | Nodular | Curettage | 42 | Surgical excision |
Among surgical excision patients (n = 59), follow-up data was available for 49 (median: 40 months, range: 6–57 months). Of these, 45 remained recurrence-free, two experienced site-specific recurrences and two died of causes unrelated to the disease or intervention. Mohs micrographic surgery patients (n = 4) had no recurrences but had shorter follow-up (median: 9.5 months, range: 8–10 months). Mean disease-free survival favoured surgery (55.58 ± 0.98 months) over medical or destructive therapies (43.6 ± 3.482 months, p = 0.003, [Figure 6]). The medical/destructive group however had more patients with multiple lesions (p = 0.003) and associated genodermatoses (p < 0.001).
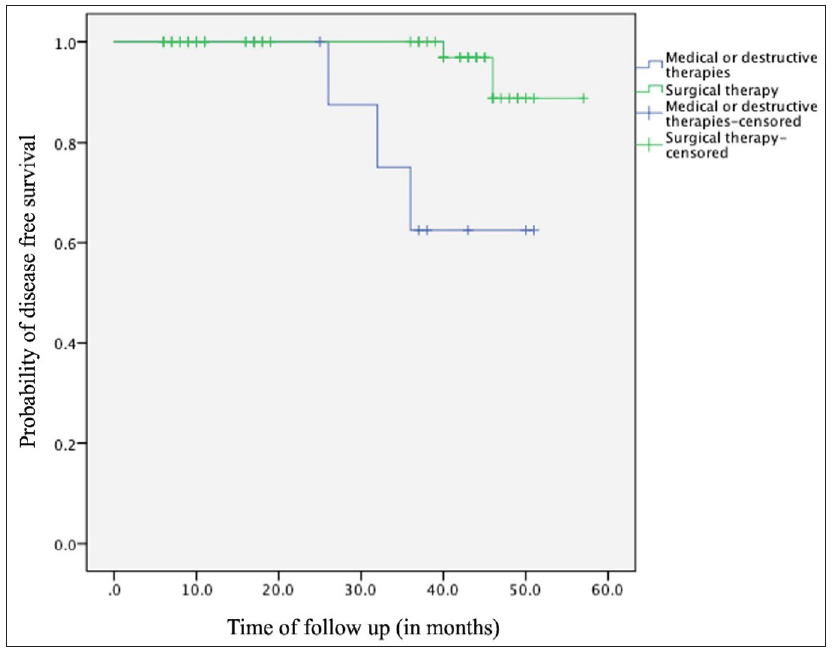
- Kaplan–Meier survival analysis depicting disease-free survival between surgically treated and medically/destructively treated patients over the follow-up duration.
We used Cox proportional hazards regression to assess the impact of various factors on recurrence risk. None of these variables, including age (HR: 0.97, p = 0.219), gender (HR: 0.39, p = 0.388), lesion duration (HR: 0.99, p = 0.822), size (HR: 5.27, p = 0.197), number (HR: 1.28, p = 0.844) and treatment type (medical/destructive or surgical) (HR: 0.19, p = 0.263), were statistically significant. This suggests their limited influence on recurrence risk, possibly due to the small number of recurrences observed.
Discussion
BCC has seen rising incidence due to factors like an ageing population, increased sun exposure, and lifestyle changes. Asian BCC tends to differ from the Western type with lower incidence, older age at first lesion, fewer total lesions, and more pigmented lesions.16–18
In our study population, the age and gender distribution aligned with prior Indian studies.11–15 BCC was most common in the seventh decade of life, with only three patients younger than 20, all having Xeroderma pigmentosum (XP). A female predominance was noted, possibly due to intermittent sun exposure. The infrequent use of sun protection measures in our study highlights the need for enhanced sun safety awareness and education.19
Our results show that BCC primarily affects the cheeks, followed by the nose and periorbital region, consistent with prior studies from India, Korea, and Japan.11,20,21 Nodular BCC was the predominant subtype in patients with darker skin phototypes, in line with prior research on BCC in this population.11,15,18
In a systematic review examining dermoscopy in BCC cases, pigment structures were observed in only 36% of the cases.22 In our study, focusing on phototypes III, IV, and V, over half the patients exhibited pigment structures, with clinical pigmentation observed in 45.2% of cases, aligning with prior research in Asian populations with consistent presence of pigment structures.11,23 Pigment structures enhance the sensitivity and specificity of dermoscopy in pigmented BCC cases.24 Further, pigmented BCCs have a lower risk of recurrence following surgical excision since the tumour margins are better delineated.25
During follow-up, five of seventy patients developed new BCCs at different sites, including two XP patients. The recurrence of primary lesions was observed in five cases, including two that managed with surgical excision. Notably, patients experiencing a recurrence of lesions had high risk factors, including lesions on H areas [high risk for recurrence: “mask areas” of face (central face, eyelids, eyebrows, periorbital, nose, lips, chin, mandible, preauricular and postauricular skin/sulci, ear, temple), genitalia, hands and feet], basosquamous histopathological subtypes, or prior recurrence [Table 3].
Strengths of this study include extensive data collection, robust inclusion criteria, utilisation of dermoscopy and histopathology for accurate diagnosis, and availability of follow-up information.
Limitations
Limitations include retrospective study design, potential for incomplete data, selection bias, and relatively small sample size. Follow-up duration was short, however, as nearly two-thirds of recurrences are evident in the first 3 years after the intervention,6 a significant proportion of recurrences was likely recorded.
Conclusion
The lack of sun protection and delayed attention highlight the need for heightened awareness of BCC among individuals with darker skin tones. Treatment outcomes are positive, with rare recurrences in the absence of high-risk factors. Vigilant follow-up is crucial, particularly for those with prior malignancies or sun exposure. Surgical excision remains the primary treatment, with customisation based on lesion characteristics recommended. Patients with genodermatoses or immunosuppression require specialised care.
Ethical approval
Given that this study was a retrospective chart review during which we anonymized patient data at the point of collection, a waiver of consent from the institutional ethics committee was sought.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Key statistics for basal and squamous cell skin cancers. Accessed May 18, 2023
- Environment, phenotype and genetics: risk factors associated with BCC of the skin. Expert Rev Anticancer Ther. 2002;2:570-9.
- [CrossRef] [PubMed] [Google Scholar]
- Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178:890-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of Dermoscopy in the assessment of basal cell carcinoma. Front Med (Lausanne). 2021;8:718855.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnosis and treatment of basal cell carcinoma: European consensus based interdisciplinary guidelines. Eur J Cancer.. 2019;118:10-34.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-28.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell skin carcinoma and other nonmelanoma skin cancers in Finland from 1956 through 1995. Arch Dermatol. 1999;135:781-6.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of basal and squamous cell carcinomas of the skin in Sion, Switzerland: A case-control study. Tumori. 1999;85:435-42.
- [CrossRef] [PubMed] [Google Scholar]
- Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-32.
- [CrossRef] [PubMed] [Google Scholar]
- Increased proportion of aggressive-growth basal cell carcinoma in the veterans affairs population of palo alto, California. J Am Acad Dermatol. 1996;35:907-10.
- [CrossRef] [PubMed] [Google Scholar]
- A multicentric study on dermoscopic patterns and clinical-dermoscopic-histological correlates of basal cell carcinoma in Indian skin. Clin Exp Dermatol. 2022;47:1982-90.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma-clinico-pathological study in Eastern India in correlation with different risk factors. Indian J Pathol Microbiol. 2022;65:869-72.
- [CrossRef] [PubMed] [Google Scholar]
- A study of Basal cell carcinoma in South asians for risk factor and clinicopathological characterization: a hospital based study. J Skin Cancer. 2014;2014:173582.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Basal cell carcinoma: A 6-year clinicopathological study from the Sub-Himalayan Region of North India. CHRISMED J Health Res. 2019;6:254-8.
- [CrossRef] [Google Scholar]
- Basal cell carcinoma in the North Indian population: clinicopathologic review and immunohistochemical analysis. Indian J Dermatol Venereol Leprol. 2011;77:328-30.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma in asians: a retrospective analysis of ten patients. J Skin Cancer. 2012;2012:741397.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Basal cell carcinoma in Singapore: a prospective study on epidemiology and clinicopathological characteristics with a secondary comparative analysis between Singaporean Chinese and Caucasian patients. Australas J Dermatol. 2015;56:175-9.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma--epidemiology from 269 cases. J Eur Acad Dermatol Venereol. 2010;24:1359-60.
- [CrossRef] [PubMed] [Google Scholar]
- Facial site distribution of basal cell carcinoma in Japanese. Exp Dermatol. 2019;28:69-71.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of basal cell carcinoma and squamous cell carcinoma by facial esthetic unit. Arch Plast Surg. 2013;40:387-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dermoscopic features of basal cell carcinoma and its subtypes: A systematic review. J Am Acad Dermatol. 2021;85:653-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recent reductions in the size of facial pigmented basal cell carcinoma at diagnosis and the surgical margin: A retrospective and comparative study. J Dermatol. 2021;48:661-6.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnostic accuracy of dermoscopy for basal cell carcinoma: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmentation of basal cell carcinoma is inversely associated with tumor aggressiveness in Asian patients. J Am Acad Dermatol. 2019;80:1755-7.
- [CrossRef] [PubMed] [Google Scholar]







