Translate this page into:
Co-existent acquired perforating collagenosis and lepromatous leprosy with erythema nodosum leprosum: Response to treatment
2 Department of Pathology, University College of Medical Sciences and GTB Hospital, University of Delhi, Delhi-110 092, India
Correspondence Address:
Archana Singal
B-14, Law Apartments, Karkardooma, Delhi-110092
India
| How to cite this article: Gupta L, Singal A, Pandhi D, Sharma S. Co-existent acquired perforating collagenosis and lepromatous leprosy with erythema nodosum leprosum: Response to treatment. Indian J Dermatol Venereol Leprol 2011;77:520-522 |
Sir,
Acquired perforating collagenosis (PC) is an uncommon, benign perforating dermatosis of multifactorial etiology in which the collagen is extruded through the epidermis. It presents as skin colored or erythematous papules with central plugging. Inherited and acquired forms exist. The latter presents in adulthood, and is associated with systemic disorders [1] such as diabetes and renal disease in majority of the cases. We report a the case of lepromatous leprosy with erythema nodosum leprosum (ENL) along with PC lesions that resolved following antileprosy treatment and treatment of reaction.
A 60-year-old man presented with multiple erythematous papules and plaques on the face, trunk, and on proximal limbs of 6 months duration. Four months later he developed recurrent crops of multiple erythematous, painful papulo-nodular lesions that resolved spontaneously within 3-5 days with cyanotic discoloration. In addition, the patient reported multiple, pruritic, persistent, umbilicated lesions interspersed with the papulo-nodular lesions [Figure - 1]. He had paresthesia of hands and feet, unnoticed burns and slippage of footwear. There was no history of trauma. Similar complaints were not present in the family members.
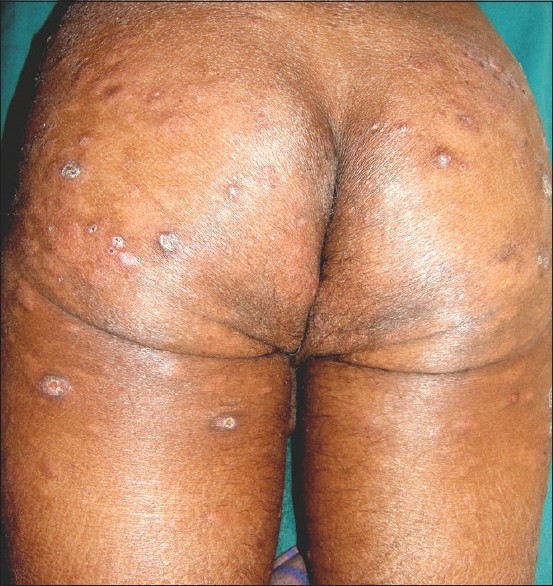 |
| Figure 1: Perforating collagenosis lesions interspersed with ENL lesions on the background of diffuse infiltration over the buttock before treatment |
Dermatological examination revealed diffuse infiltration of the face, ear lobules and buttocks and multiple, erythematous ill-defined minimally anesthetic plaques over face, trunk, and proximal limbs. Bilateral greater auricular, ulnar, radial cutaneous, common peroneal, and posterior tibial nerves were enlarged and nontender. Sensory examination revealed glove and stocking anesthesia up to knees and elbows. Clawing of toes was present.
Hematological investigations including complete hemogram, liver and kidney function tests including creatinine clearance, and X-ray chest were within normal limits. Slit skin smear was positive for acid-fast bacilli with a bacteriological index (BI) of 4+. Skin biopsy from the tender nodule demonstrated sheets of foamy macrophages and neutrophils in the dermis along with necrotizing vasculitis affecting the venules and capillaries and foci of collagen necrosis in the dermis, consistent with the diagnosis of lepromatous leprosy with the erythema nodosum leprosum. Biopsy from the umbilicated papule demonstrated epidermal hyperkeratosis, acanthosis and, follicular and extrafollicular plugging. The plugs extended up to the upper dermis forming vertical channels filled with parakeratotic keratin, collagen, and pyknotic nuclei [Figure - 2]. The collagen in the dermis demonstrated basophilia which stained red with van Gieson′s stain corroborating the clinical diagnosis of PC. Thus the patient was labeled to have lepromatous leprosy (LL) with ENL with coexistent PC lesions and was started on multibacillary multidrug therapy. Clofazimine 100 mg thrice daily and oral paracetamol 500 mg thrice daily were administered for the management of reaction along with liquid paraffin topically. Two month following treatment, the ENL lesions resolved completely. New PC lesions ceased to appear, while majority of the older ones healed with atrophic scarring [Figure - 3].
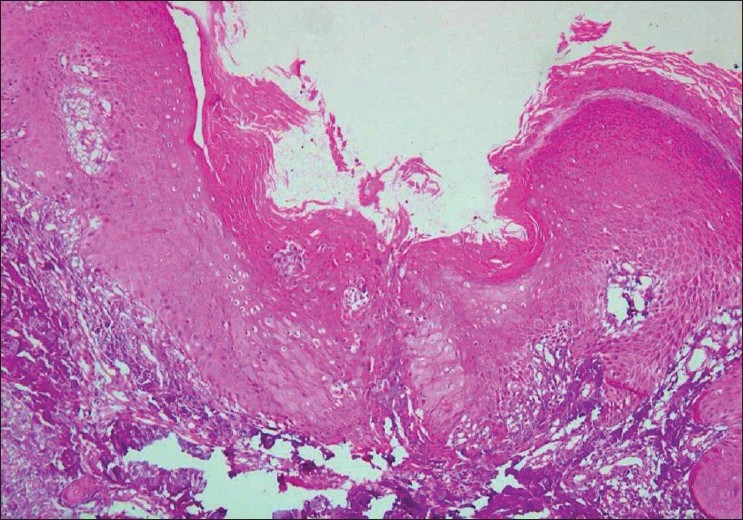 |
| Figure 2: Skin biopsy from perforating collagenosis lesion showing epidermal hyperkeratosis, acanthosis, and transepidermal elimination of collagen fibres from the defect in the epidermis as shown by the arrow (H and E, ×100) |
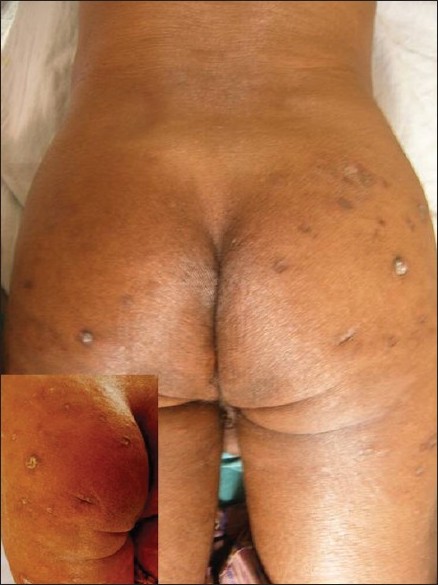 |
| Figure 3: Post-treatment, most perforating skin lesions healing with atrophic scars with a closer view of the same in the inset |
Transepidermal elimination disorder is characterized by elimination of foreign material from the corium by upward movement of regenerating epithelial cells. PC is a perforating disorder characterized by extrusion of collagen. Acquired PC is most commonly associated with diabetes and renal disease. [1] Dermatological pruritic disorders such as scabies, atopic dermatitis, insect bites, and lichen amyloidosis have been anecdotally reported with PC.
In genetically predisposed individuals, trauma in the form of intense pruritus and subsequent scratching is a common inciting factor for cutaneous and histopathological changes. [2] Trauma leads to release of matrix metalloproteinases (MMP) and serine proteases which transgress the epithelium and digest the extra cellular matrix components contributing to the formation of perforating lesions. [3] This may also lead to a damage in the basement membrane structures by cleaving the anchoring fibrils and collagen IV. [4] The infiltrating leucocytes release various cytokines in the surrounding tissue, such as IL-1 and TGF-beta, which are important modifiers of MMP activation.
Coexistent PC and leprosy has been reported on very few occasions. Patki et al.[5] reported two cases of coexistent leprosy with reactive PC. [5] In the present case, PC lesions were widespread, involving the proximal extremities and trunk interspersed among the lepromatous nodules and ENL lesions where trauma could not be incriminated as an inciting factor. These lesions were present simultaneously at the time of the patient′s first visit.
ENL is an immune complex phenomenon in which fibrinoid degeneration of the collagen and elastic fibers occurs. [6] Probably, in genetically predisposed individuals inflammatory insult occurring in type II lepra reaction results in the release of cytokines and other inflammatory mediators that damage the dermal collagen including collagen at the basement membrane zone, initiating the development of perforating skin lesions. This hypothesis is further supported in the study by Herzinger et al.[7] who observed extrusion of type IV collagen by special staining techniques. [7] Improvement and subsidence of these lesions following treatment of leprosy and of reaction corroborate the aforesaid hypothesis.
| 1. |
Mehregan AH, Schwartz OD, Livingood CS. Reactive perforating collagenosis. Arch Dermatol 1967;96:277-82.
[Google Scholar]
|
| 2. |
Poliak SC, Lebwohl MG, Parris A, Prioleau PG. Reactive perforating collagenosis associated with diabetes mellitus. N Engl J Med 1982;306:81-4.
[Google Scholar]
|
| 3. |
Patterson JW, Brown PC. Ultrastuctural changes in acquired perforating dermatosis. Int J Dermatol 1992;31:201-5.
[Google Scholar]
|
| 4. |
Briggaman RA, Schechter NM, Fraki J, Lazarus GS. Degradation of the epidermal-dermal junction by proteolytic enzymes from human skin and human polymorphonuclear leukocytes. J Exp Med 1984;160:1027-42.
[Google Scholar]
|
| 5. |
Patki AH, Mehta JM. Coexistent lepromatous leprosy and reactive perforating collagenosis. Cutis 1991;48:137-40.
[Google Scholar]
|
| 6. |
Ridley MJ, Ridley DS. The immunopathology of erythema nodosum leprosum: The role of extravascular complexes. Lepr Rev 1983;54:95-107.
[Google Scholar]
|
| 7. |
Herzinger T, Schirren CG, Sander CA, Jansen T, Kind P. Reactive perforating collagenosis-transepidermal elimination of type IV collagen. Clin Exp Dermatol 1996;21:279-82.
[Google Scholar]
|
Fulltext Views
2,759
PDF downloads
2,133





