Translate this page into:
Cold agglutinin disease-associated digital gangrene treated with plasmapheresis
Correspondence Address:
Atsushi Utani
Department of Dermatology, Nagasaki University Graduate School of Biomedical Sciences, 1-12-4 Sakamoto, Nagasaki 852-8523
Japan
| How to cite this article: Koike Y, Akiyama Y, Utani A. Cold agglutinin disease-associated digital gangrene treated with plasmapheresis . Indian J Dermatol Venereol Leprol 2014;80:575-576 |
Sir,
Cold agglutinin disease (CAD) is a type of autoimmune hemolytic anemia. Cold agglutinins are usually immunoglobulin M (IgM) antibodies that bind to erythrocyte surface antigens at low temperatures. Primary cold agglutinin disease is characterized by synthesis of kappa monoclonal IgM antibodies by proliferating clonal B-lymphocytes whereas in secondary disease, synthesis of polyclonal IgM cold agglutinins accompanies acute infections and malignant lymphoma. More than 90% of patients have cutaneous lesions such as reversible acrocyanosis, Raynaud′s phenomenon, and livedo reticularis. [1] Since agglutination is rapidly reversible on warming, cold agglutinins rarely cause permanent obstruction of blood vessels leading to digital gangrene. [2],[3],[4]
A 54-year-old Japanese man had observed transient digital color changes during each cold season of the past decade. He had no history of underlying diseases, infections, or trauma. In January 2011, his fingers and toes discolored rapidly to gray-black. This was associated with sharp pain which had started 1 week earlier. A physical examination on admission revealed that several fingers and toes were cyanotic and some of the digits had dry gangrene along with marked peripheral coldness [Figure - 1]a-c. Routine laboratory investigations indicated moderate hemolytic anemia (hemoglobin level of 8.3 g/dL, unconjugated bilirubin level of 1.3 mg/dL), elevated lactate dehydrogenase (LDH) levels (1,056 IU/L) and increased total IgM levels (456 mg/dL), and revealed a fasting sugar level of 278 mg/dL, then diagnosed as poorly controlled type 2 diabetes (T2DM). A coagulation study including cryoglobulins and cryofibinogen revealed that blood clotting was unaffected. A direct Coombs test showed that C3b and C3d were bound to erythrocyte surface antigens. In addition, cold agglutinin tests revealed elevated titers of 1:1,024 at 4˚C and 1:512 at 12˚C, while no agglutination was detected at 25˚C. Serological studies for viral infections and autoantibodies associated with collagen disease were negative. No malignancy was identified using computed tomography (CT). Monoclonal IgM-kappa protein was detected by immunoelectrophoretic analysis of the patient′s serum. Consequently, primary cold agglutinin disease was diagnosed. Bone marrow aspiration showed discrete hyperplasia but no signs of lymphoproliferative disorders. A peripheral blood smear showed clumping of adherent erythrocytes [Figure - 1]d. A skin biopsy from a discolored finger revealed thromboembolic changes in capillaries located in the uppermost dermis and endothelial proliferation associated with thrombosis in the subcutaneous tissue [Figure - 1]e and f.
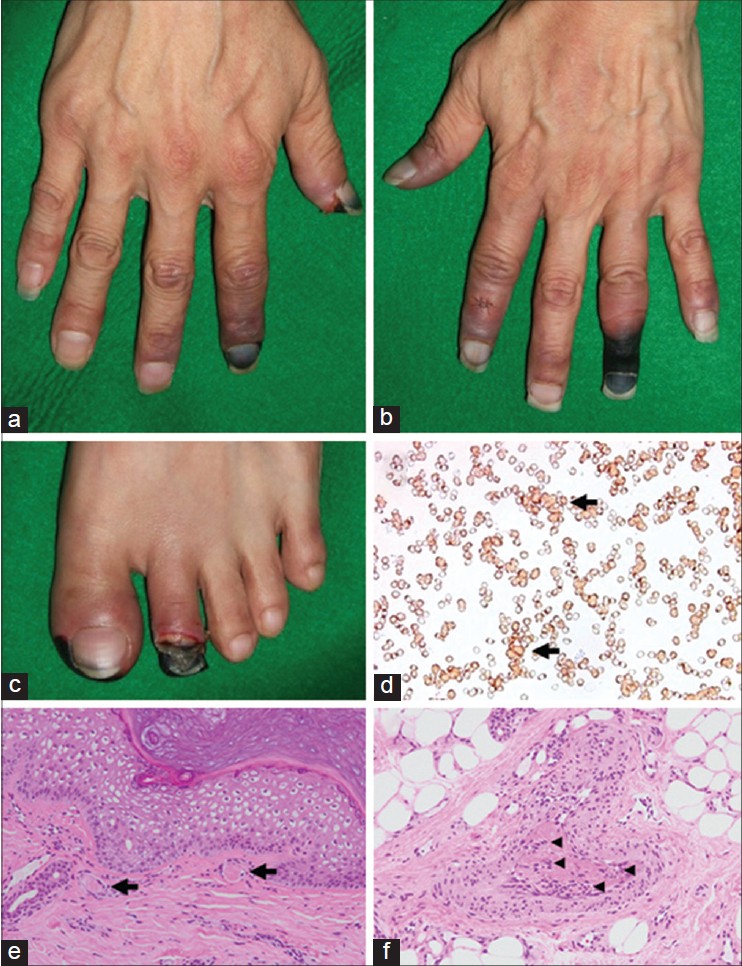 |
| Figure 1: (a-c) Clinical features of the CAD patient. Acrocyanosis and digital gangrene rapidly appeared in the patient's hands and feet. (d) A blood smear test revealed erythrocytic coagulation. The arrow indicates a representative agglutination. Histopathological examination of the cyanotic lesion on the finger revealed thrombosis (arrow) in superficial dermal vessels (e) and endothelial proliferation (arrowhead) associated with thrombosis in subcutaneous vessels (f). (e and f: H and E, ×200) |
Although the patient′s extremities were kept warm and treated with vasodilating agents, new gangrenous lesions appeared on the fingers. Weekly double-filtration plasmapheresis (DFPP) up to 3,000 ml of plasma per treatment using cascadeflo EC-50W was started to reduce cold agglutinins. After commencing plasmapheresis, total IgM levels rapidly decreased to half or less and cold agglutinin titers at 4˚C dropped from 1 in 1024 to 1:128. Further gangrene did not develop and autoimmune hemolysis was no longer detected. In all, nine sessions of double-filtration plasmapheresis was undertaken. Finally, debridement and reepithelialization of the gangrenous areas was achieved.
It is known that cold agglutinin disease rarely leads to digital gangrene. However, in the present case it can be postulated that the patient′s untreated type 2 diabetes mellitus may have, at least in part, precipitated insufficient circulation due to cold agglutinin disease-induced thromboembolism. Therefore, cold agglutinin disease should be recognized as a risk factor for digital gangrene when it is present in a patient with diabetes. A previous report has suggested that glomeruloid reactive angioendotheliomatosis is a distinct histological feature of the necrotic lesions seen in cold agglutinin disease. [2] This is consistent with the thrombosis-associated endothelial proliferation observed in the subcutaneous tissue of our patient. Treatment for the disease depends on its etiology and severity. Rigorous avoidance of cold exposure is generally considered to be effective treatment for cutaneous lesions. [2],[3] Vasodilating agents can also be used to facilitate passage of blood clots through peripheral vessels but were not very effective in our patient. In contrast, antiplatelet agents or anticoagulant drugs were not employed because cold agglutination does not require platelets and clotting factors. In primary cold agglutinin disease, not all patients require pharmacological therapy and immunosuppressive agents such as corticosteroids are almost ineffective. [1] The most efficient therapy is administration of fludarabine and rituximab in combination, although toxicity may be a concern. [5] In the future, double-filtration plasmapheresis which is expected to directly reduce the cold agglutination and to improve clinical symptoms should be studied in greater detail as a treatment for acute exacerbations of cold agglutinin disease with high titers of cold agglutinin.
| 1. |
Berentsen S, Tjonnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev 2012;26:107-15.
[Google Scholar]
|
| 2. |
Porras-Luque JI, Fernandez-Herrera J, Dauden E, Fraga J, Fernandez-Villalta MJ, Garcia-Diez A. Cutaneous necrosis by cold agglutinins associated with glomeruloid reactive angioendotheliomatosis. Br J Dermatol 1998;139:1068-72.
[Google Scholar]
|
| 3. |
Ciejka JZ, Cook EB, Lawler D, Martin J, Woodson RD, Graziano F. Severe cold agglutinin disease and cryoglobulinemia secondary to a monoclonal anti-Pr2 IgM lambda cryoagglutinin. Clin Exp Rheumatol 1999;17:227-31.
[Google Scholar]
|
| 4. |
Jeskowiak A, Goerge T. Images in clinical medicine. Cutaneous necrosis associated with cold agglutinins. N Engl J Med 2013;369:e1.
[Google Scholar]
|
| 5. |
Berentsen S, Randen U, Vagan AM, Hjorth-Hansen H, Vik A, Dalgaard J, et al. High response rate and durable remissions following fludarabine and rituximab combination therapy for chronic cold agglutinin disease. Blood 2010;116:3180-4.
[Google Scholar]
|
Fulltext Views
6,622
PDF downloads
1,335





