Translate this page into:
Comparative biorelease study of fluticasone in combination with antibacterial (Neomycin) and or antifungal (coltrimazol, miconazole) agents by histamine percutaneous reaction method in healthy volunteers
Correspondence Address:
K Sharma
From Medical Services Glaxo (I) Ltd., Mumbai
India
| How to cite this article: Shahani S R, Jerajani H R, Sharma K, Cooverji N D. Comparative biorelease study of fluticasone in combination with antibacterial (Neomycin) and or antifungal (coltrimazol, miconazole) agents by histamine percutaneous reaction method in healthy volunteers. Indian J Dermatol Venereol Leprol 1997;63:173-177 |
Abstract
Fluticasone propionate is a novel, potent and topically active synthetic corticosteroid preparation with a much reduced potential, in relation to its anti-inflammatory potency, for unwanted systemic side effects. It is indicated for the treatment of eczema, dermatitis etc. The objective of the present study was to evaluate and compare the biorelease of fluticassone with placebo (base formulation); its combination with antifungal (miconazole, clotrimazole) and / or antibacterial agents based on the attenuation of histamine induced wheal and flare reaction and flare intensity (on visual analouge scale) by McNemar test. In the present study, fluticasone alone and in combination with clotrimazole, miconazole and neomycin significantly reduced the wheal and flare response of histamine prick test. The flare intensity response was also significantly inhibited by these treatments. Furthermore, there was no difference in the anti-inflammatory activity of various treatment groups. It may, therefore, be concluded that antibacterial (neomycin) and / or antifungal (miconazole, clotrimazole) agents in combination with steroid (fluticasone) do not alter the pharmacodynamic response of the latter.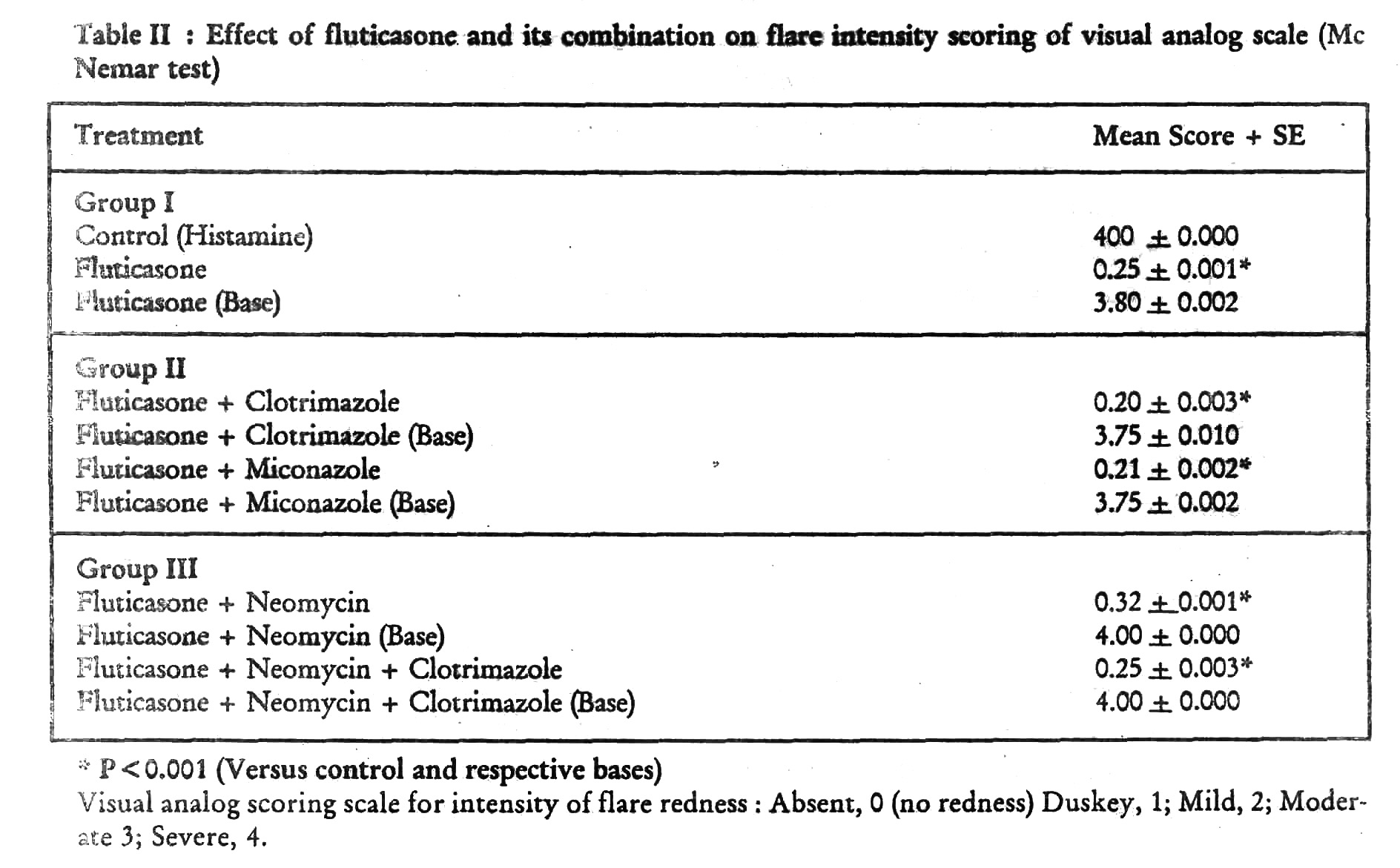


Topical corticosteroids when applied on a normal skin under occlusion produce blanching at the site of occlusion due to their vaso-constrictive activity. A comparison based on the blanching effect of steroids is the commonly used technique to grade the potency of steroids. This methodology has been further improved by carrying out histamine prick tests at the site of the blanching.[1] Histamine is known to be an important mediator of allergic and inflammatory responses and in conditions involving an increase in vascular permeability.[2] Depending upon the potency and availability of the topical steroid at a particular site, the wheal and flare (erythema) response produced by histamine can be objectively measured. In general, the more potent the topical steroid, the less severe is the histamine response and vice versa. The method, therefore is a simple, reliable and reproducible procedure.
Fluticasone propionate is a novel, potent and topically active synthetic corticosteroid preparation with a much reduced potential, in relation to its anti-inflammatory potency, for unwanted systemic side effects. It is indicated for the treatment of eczema, dermatitis etc.[3]
The objective of the present study was to evaluate and compare the biorelease of fluticasone with placebo (base formulation); its combination with antifungal (miconazole, clotrimazole) and/of antibacterial agents (neomycin) based on the attenuation of histamine induced wheal and flare reaction and flare intensity (on visual analouge scale) by Mc Nemar test.
Subjects and Methods
The study was carried out on 24 healthy adult volunteers aged between 20 and 40 years. None of the volunteers had used any systemic or topical corticosteroid / antihistamine for atleast 3 weeks prior to their inclusion. All subjects were willing to participate in the study and gave written informed consent.
Study design
The study was randomized, open controlled and comparative volunteer trial. Volunteers were divided into 3 groups of 8 volunteers in each group. They were studied in the groups of 12 per day on two different days. The groupings were done as follows:
Group I Fluticasone plain, histamine alone and fluticasone base.
Group II Fluticasone + clotrimazole, fluticasone + miconazole and their respective bases.
Group III Fluticasone + clotrimazole + neomycin; fluticasone + neomycin and their respective bases.
Fluticasone + clotrimazole and fluticasone + clotrimazole + neomycin had similar base composition whereas all other regimes had different base compositions.
Occlusion procedure
The volunteers were instructed to arrive in the clinical pharmacology laboratory at 3.00 p.m. Standard quantities (0.1 ml) of the topical steroid combinations were applied in random fashion to the volar surface of the forearm. The sites were occluded for 18 hours. Thereafter, next day at 9.00 a.m., the occlusion was removed and sites were cleaned with soap and water.
Histamine test
Before carrying out the histamine percutaneous reaction method, a gap of about half an hour was allowed till the erythema due to the adhesive tape was cleared. Following that, each subject received an intradermal injection of histamine acid phosphate 2 μg/0.1 ml in sterile distilled water on the flexor surface of the forearm with the subject lying in supine position. Blood pressure with sphygmomanometer and pulse rate was recorded in each subject before and after the injection of histamine.
Fifteen minutes after the injection of histamine, the area of wheal and boundaries of erythema were measured by marking the area with a marking pen. This was transferred on to a transparent tape and traced on a transparent butter paper. The butter paper was placed on a graph paper and the areas of wheal and erythema were measured in square mm. The wheal area and erythema area were calculated in square mm from the graph paper. The duration of itch response was noted. A visual analouge scale for severity of flare response was prepared and alteration after each treatment was assessed by the same investigator throughout the study. Intradermal injection was also given by the same investigator throughout the study.
Spontaneously reported side effects were recorded and each subject was specifically asked about the side effects, if any.
Analysis of results
On completion of the study, the area of the wheal and erythema for each treatment for individual subject was calculated and mean values for each treatment schedule were found out. Paired student′s ′t′ test was employed for statistical analysis of different treatment on histamine wheal and erythema. For subjective parameter i.e. flare intensity on visual analouge scale, Mc Nemar test was employed.
Results
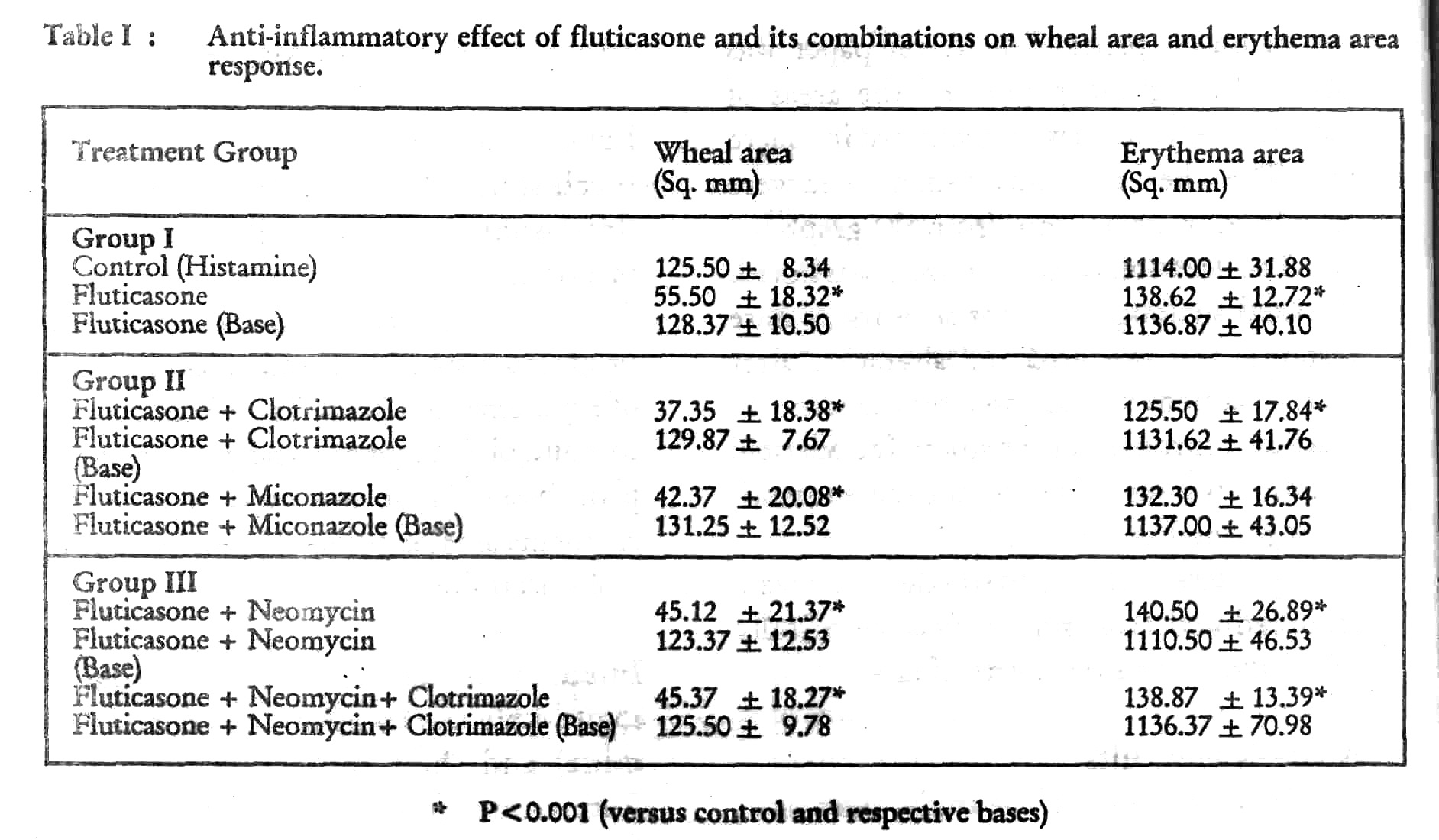
Wheal area response Pretreatment (occlusion) of subjects with fluticasone, fluticasone + clotrimazole, fluticasone + miconazole, fluticasone + neomycin, fluticasone + neomycin + clotrimazole produced a significant reduction in the size of wheal (p< 0.001; [Table - 1]) as compared to control (histamine) and their respective plain bases. There was no difference in the wheal response of plain bases vs control (histamine).
Erythema area response Pretreatment (occlusion) of subjects with fluticasone, fluticasone + clotrimazole, fluticasone + miconazole, fluticasone + neomycia, fluticasone + neoraycine + clotrimazole produced a significant reduction in the area of erythema (p < 0.001 [Table - 1]) as compared to control (histamine) and their respective plain bases. There was no difference in the erythema area response of plain bases vs control (histamine.)
Intensity of flare response on visual analogue scale As compared to control (histamine) and their respective plain bases, fluticasone, fluticasone + clotrimazole, fluticasone + miconazole, fluticasone + neomycin and fluticasone + clotrimazole + neomycin showed a significant reduction in flare response (p < 0.001, [Table - 2]). There was no difference in the flare response of control (histamine) vs plain bases.
Blanching at the site of occlusion was produced by all the active treatment groups. No attempt, however, was made to score the extent of blanching with various formulations. None of the subjects reported any side effects after occlusion with fluticasone and its various combinations.
Discussion
The role of topical steroids in inflammatory dermatoses as a potent weapon has been well established for a considerable period of time.[4] For the screening and evaluation of topical steroids, a variety of methods viz. cotton or carrageenan pellet test or granuloma pouch test (in animals); inhibition of artificial inflammation (UV, oxazolone, pyrogen induced erythema) and blanching tests (in humans) are employed.[5] Among these, vasoconstrictor assay developed by McKenzie and Stoughton was considered as one of the best till recently. The action of histamine on skin is known to be manifested by the classical triple response.[6] An intrad-ermal injection of histamine leads to oedema (wheal) and erythema. Erythema is initially localized at the site of injection due to direct vasodilatation but spreads rapidly due to axon reflex resulting in the characteristic wheal, erythema and flare.
The histamine bioassay of topical steroids has many advantages over the other known assay techniques. It involves the suppression of the wheal produced by histamine, therefore, it is more likely to be nearer the clinical situation than vasoconstrictor assay which demonstrates the effect on normal blood vessels. Further on a dark skin, there is considerable difficulty in appreciating the pallor produced by local vasoconstruction.[4]
The assay is a simple, reliable and reproducible procedure. More than two or three compounds may be tested at a time on a single subject and hence the study may be conducted even with a smaller number of test subjects. The end point of the assay is very easy to observe. Skin colours also do not interfere with the interpretation of the results. Although it is non-clinical human assay, it resembles clinical conditions closely having some similarity to the process involved in the development of inflammatory dermatoses,[1] and has been used for the evaluation of the relative potencies of topical steroids.[4]
In the present study, fluticasone alone and in combination with clotrimazole, miconazole and neomycin significantly reduced the wheal and flare response of histamine prick test. The flare intensity response was also significantly inhibited by these treatments. Furthermore, there was no difference in the antiinflammatory activity of various treatment groups. It may, therefore, be concluded that antibacterial (neomycin) and /or antifungal (miconazole, clotrimazole) agents in combination with steroid (fluticasone) do not alter the pharmacodynamic response of the latter.
| 1. |
Reddy B S N, Singh C. A new model for human bioassay of topical coitlcosteroids, Br J Dermatol, 1976;94:191-193.
[Google Scholar]
|
| 2. |
Chauhan C K, Shahani S R. Antihistaminic efficacy of ranitidine with and without dimethendine maleate on histamine induced cutaneous reaction, Ind J Med Res (B) 1992;96:128-132.
[Google Scholar]
|
| 3. |
Polano M K In: Topical Skin Therapeutics, Editor, Polano, Churchill Livingstone, London, 1984;126-128.
[Google Scholar]
|
| 4. |
Lewis T. The blood vessels of the human skin and their responses, Show et Sons Ltd. London, 1927:10.
[Google Scholar]
|
| 5. |
Singh G. Evaluation of three commercially formulated topical steroids using a new bioassay technique, The Ind Pract 1983;36:241-245.
[Google Scholar]
|
| 6. |
Harding S M. The human pharmacology of fluticasone propionate, Resp Med, 1990;84 (Suppl. IA):25-29.
[Google Scholar]
|





