Translate this page into:
Comparison of anti-desmoglein antibodies titres and anti-M3 muscarinic acetylcholine receptor antibody titres as biomarker for potential pemphigus between subjects who are occupationally exposed and not exposed to pesticides
Corresponding author: Dr. Dipankar De, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. dr_dipankar_de@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: De D, Kamat D, Kaur M, Kumari D, Pal A, Handa S, et al. Comparison of anti-desmoglein antibodies titres and anti-M3 muscarinic acetylcholine receptor antibody titres as biomarker for potential pemphigus between subjects who are occupationally exposed and not exposed to pesticides. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_256_2024
Abstract
Background
The pathogenesis of pemphigus is multifactorial, and pesticides have been implicated as one of the triggering factors due to their interaction with acetylcholine receptors.
Aim
The aim of this study was to observe the effect of exposure to pesticides (by blood pesticide levels) on serological markers of pemphigus (anti-Dsg1, anti-Dsg3, and anti-M3 ACh receptor antibodies) amongst healthy individuals involved in pesticide use.
Methods
This was an observational, cross-sectional pilot study performed in Punjab. The study subjects were 45 couples (husband and wife) wherein the husband was involved in activities like spraying and handling pesticides, and the wife was considered a non-genetically related control (not occupationally exposed to pesticides). Clinical data were collected by a structured questionnaire, and venous blood was collected to test blood pesticide levels. Gas chromatography - mass spectroscopy (GC-MS) and enzyme-linked immunosorbent assay (ELISA) were used for anti-Dsg-1, anti-Dsg-3, and anti-M3Ach receptor antibody level detection.
Results
Pesticides were detected in both husband and wife. There was no correlation between the duration of pesticide exposure and their blood levels. Chlorpyrifos was most frequently detected in husbands and β- endosulfan in wives. The levels of anti-Dsg-1 and anti-Dsg-3 titres were below the diagnostic cut-off values (20 RU/mL) for pemphigus in all participants. Husbands had significantly higher anti-Dsg3 values (p=0.029). There was no statistically significant association between the presence of pesticides and anti-Dsg-1, anti-Dsg-3, and anti-M3 ACh receptor antibodies in the sera. Anti-Dsg-1 antibodies had a significant positive correlation with chlorpyrifos serum levels (r= 0.3, p=0.005).
Limitation
Determining aspects like expression of adhesion proteins on keratinocyte cell lines, long term follow up of healthy subjects and correlation with pesticide levels with cases of pemphigus was not done as it is cost intensive.
Conclusion
The exposure to pesticides did not significantly increase any of the biomarkers above clinical cut-off levels in any of the healthy participants. Considering the rarity of clinically manifest pemphigus, many healthy subjects need to be studied with the current study design. Alternatively, the expression of adhesion proteins or their adhesion function can be assessed in keratinocyte cell lines upon incubation with varying concentrations of pesticides.
Keywords
Pesticides
pemphigus
assessment of causality
pilot study
Introduction
Pemphigus results from the interplay between genetic and environmental factors. Genetic susceptibility to pemphigus has been demonstrated by allelic and haplotype studies. Distinct HLA (Human Leukocyte Antigen) class II alleles determine the autoimmune response to desmoglein 3 in patients with pemphigus.1
The acronym PEMPHIGUS represents the environmental factors that are considered important in pemphigus pathogenesis. It stands for PEsticides, Malignancy, Pharmaceuticals, Hormones, Infectious agents, Gastronomy, Ultraviolet radiation and Stress. Pesticides have been proposed to be involved in the pathogenesis of pemphigus.2
Organophosphate pesticides inhibit acetylcholinesterase, causing accumulation of acetylcholine at cholinergic receptors. In cases of long exposure, individuals develop tolerance, resulting in a decline in the density of nicotinic and muscarinic receptors. In case of such a reaction in the skin, diminished cell-cell adhesion occurs.1 Though the mechanism is biochemical acantholysis, loss of cellular adhesion may lead to exposure of cryptic desmoglein antigens, formation of autoantibodies against desmogleins, and maintenance of pemphigus in genetically predisposed individuals. Additionally, the active component of chemical compounds or their metabolites may bind to desmogleins to form haptens, which trigger T and B cell activation and autoimmunity.3
Human exposure to pesticides takes place through inhalation and dermal absorption. Production workers, formulators, sprayers, mixers, loaders, and agricultural farm workers comprise the high-risk groups with much exposure.4 Pemphigus is particularly more common in northern India.5 Though this can be attributed to genetic predisposition, it can also result due to a high-degree of pesticide exposure post the green- revolution.
We aimed to study the association between pesticide exposure and pemphigus. We analysed the effect of exposure to pesticides (as evidenced by blood pesticide levels) on the anti-desmoglein (1 and 3) and anti-muscarinic acetylcholine receptor antibody titres in healthy individuals engaged in agricultural activities, making them susceptible to occupational exposure to pesticides.
Methods
This was an observational, cross-sectional pilot study conducted in the Khera Block of Fatehgarh Sahib district of Punjab, India. An initial survey was conducted to identify individuals engaged in pesticide spraying or handling pesticides. The study subjects were couples (husband and wife), chosen by consecutive sampling based on the following selection criteria:
Inclusion criteria for the study were: (i) the couple must be living together and eating in the same household, (ii) the male member of the couple was engaged in spraying/selling/otherwise handling pesticides, (iii) the female counterpart was engaged only in household work. Exclusion criteria were: (i) known neurological disease, on treatment with cholinergic or anti-cholinergic drugs, (ii) known cases of pemphigus, first or second-degree consanguinous marriage, (iii) handling or storing pesticides in the living area of the home, which might lead to exposure of pesticides to the spouse as well and (iv) use of protective equipment as per standard recommendations while handling pesticides, thereby leading to reduced exposure. The reason for choosing couples was that the wife could be considered a non-genetically related control, not exposed to pesticides but had similar exposure to other potential causative factors like food.
Demographic details and other information like type, duration, mode of pesticide exposure, history of smoking, specific food habits, and history of drug intake, which are specifically known to trigger pemphigus were noted in the pre-structured questionnaire [supplementary file 1]. For quantifying pesticide exposure, the PEM (pesticide exposure month) index was used.6 It is calculated as PEM=No. of spraying days per year/30 × total no. of spraying years.
After obtaining informed consent, 10 mL venous blood was collected. An aliquot of 3mL was stored in a lithium/heparin vacutainer at 2-8°C.
Pesticide analysis
Chemicals and reagents
Pesticides assessed in the study were α-Hexachlorocyclohexane, β-Hexachlorocyclohexane, γ-Hexachlorocyclohexane, δ-Hexachlorocyclohexane, endrin, heptachlor, fenitrothion, aldrin, butachlor, dieldrin, p, p’ dichlorodiphenyltrichloroethane, p, p’ dichlorodiphenyldichloroethylene, o, p’ dichlorodiphenyldichloroethylene, p, p’ dichlorodiphenyldichloroethane, o, p’ dichlorodiphenyldichloroethane, α-endosulfan, β-endosulfan, chlordane, dicofol, monocrotofos, dimethoate, parathion methyl, malathion, chlorpyrifos, fenamiphos, profenophos, ethion, triazophos, phosalone, phosphamidon, tubaconazol, thiophanate methyl, and cypermethrine. Analytical reference standards of these pesticides with 99.9% purity were purchased from Sigma-Aldrich, USA and Merck, Germany. Acetonitrile (ACN), methanol, n-hexane, and distilled water of analytical grade were purchased from Thermo Fisher Scientific, USA. QuEChERS extraction kit containing salts (6 g MgSO4 and 1.5 g NaOAc) and dispersive tubes for extract clean-up (150 mg MgSO4 and 50 mg C18) were purchased from Shimadzu Analytical (India) Pvt. Ltd.
Stock solutions of 100 µg/mL pesticide standards were prepared in ethanol. Working solutions were prepared freshly by dilution. Six different concentrations ranging from 0.02 ng/mL to 2000 ng/mL were prepared for instrument calibration. The R2 values for linear calibration ranged from 0.95 to 0.99. For accuracy and quality control, recovery experiments were performed, by fortified pesticide standard from working solution in whole blood samples that are free from pesticides (duplicate samples). The signal-to-noise ratios of 3 and 10 were taken for the limit of detection (LOD), limit of quantification (LOQ), and recovery percentage. Method validation parameters value for LOD was 0.013 M, LOQ was 0.012 M, and the percentage of recovery was greater than 95%.
Sample preparation and clean-up
Pesticides were extracted from whole blood samples using the multi-residual extraction QuEChERS method. QuEChERS method (Quick, Easy, Cheap, Effective, Rugged, and Safe) provides a high recovery scope of the various chemical classes. 1 mL of ACN was added to a 4.5 mL QuEChERS extraction salt (0.5g) containing tube. Immediate dilution of 1.3 mL of distilled water in 200 µL of whole blood was mixed by vortexing method in a centrifuge. After dilution, the sample was added to a tube for extraction and immediate mixing was done by shaking with hands for 30 seconds, followed by centrifugation at 3000 rpm for 10 minutes. The organic layer was transferred to a Q-sepTM dSPE tube for evaporation under nitrogen at 60° C. Final reconstitution was done in 40 µL of n-Hexane for instrumental analysis.
Instrument used and operating condition
Gas chromatography triple quadrupole mass spectrometry (GCMS-TQ8040 Shimadzu, Japan) was used for the pesticide residue analysis. Extracted sample (5 µL) were auto-injected into the 2 mL capillary column SH-Rxi-5Sil MS (30 m long, 0.25 mm ID, 0.25 mm df). Helium (He) and argon (Ar) were used as carrier and collision gases, respectively, at 1.2 mL min-1 flow rate. The operating condition was programmed with the temperature variation: Injection temperature started from 60°C and was held for 0.8 minutes, 200°C min-1 ramp to 260°C (held for 10 minutes). The oven temperature was started from 50°C for 3 minutes, 25°C min-1 ramp to 150°C, then 5°C min-1 ramp to 200°C, 10°C min-1 ramp to 280°C (held for 20 minutes). Pesticide residue analysis in GC-MS/MS instrument was operated under the Multiple reaction monitoring mode. MRM analysis used Argon as a collision gas with 200 kPa pressure.
All the laboratory work for pesticide residue analysis (sample preparation and instrumental analysis) was done in the sophisticated instrumentation laboratory at the collaborating institution.
ELISA for serum markers of pemphigus
Serum was separated from the remaining 7 mL blood after centrifugation and was stored at -80°C up till processing with enzyme linked immunosorbent assay. The ELISA assays were done as per the manufacturer’s protocol. Since desmoglein ELISA systems (anti-Dsg-1 and anti-Dsg-3 antibodies ELISA; Euroimmun, Luebeck, Germany) have been established and widely used for diagnostic purposes, the serum was processed only one time for a given sample. The manufacturer provided cut-offs for both anti-Dsg-1 and anti-Dsg-3 antibodies ELISA were 20 RU/ mL. Anti-M3 acetyl-choline receptor antibody ELISA (Wuhan Fine Biotech, Wuhan, China) was run in duplicates, and the mean of the two values was considered for data analysis.
Statistical analysis
Descriptive and inferential statistics were used to analyse the data. Central dispersion indices were used to describe the data. Data were analysed using Spearman, Mann-Whitney, and Friedman correlation coefficients. In all tests, p ≤ 0.05 was adopted, and a two-tailed comparison was employed. Statistical analysis was performed by using the SPSS 24.0 software (IBM, USA).
Results
A total of 45 couples (90 participants) were included based on the selection criteria. The average age was 45.6 years. There were no smokers, none had any pre-existing skin conditions, and none were on any chronic medications. On questioning about food habits, no couples were found wherein one spouse would avoid a particular food of significance to the pathogenesis of pemphigus. All couples were genetically unrelated and had similar food habits.
Pesticide exposure
The mean PEM index amongst the husbands was 25.4. Analysis of blood pesticide levels was done for 43 couples. No pesticide residues were detected in sera of 53 (61.6%) participants (24 males and 29 females). At least 1 pesticide was detected in 33 (38.3%) participants (19 males, 14 females). There were 3 participants (2 males and 1 female) in whom three pesticide residues were detected. The frequency of detection of pesticides in sera was not significantly higher in males (p = 0.27). The mean blood levels for various pesticides detected are presented in Table 1. The most frequently detected pesticide was chlorpyrifos in husbands and β-endosulfan in wives. There was no significant association between the duration of pesticide exposure (PEM) and the detection of pesticides in blood (P=0.33).
| Pesticide | Number of participants | Mean blood levels (ng/mL) | |
|---|---|---|---|
| Males | Females | ||
| β- Endosulfan (OC) | 1 | 7 | 0.325 |
| Endrin (OC) | 5 | 2 | 0.084 |
| p, p-DDT (OC) | 5 | 2 | 0.157 |
| Thiophanate methyl (thiourea) | 4 | 3 | 0.139 |
| Cypermethrin (pyrethroid) | 0 | 3 | 0.045 |
| Tuboconazole (azole) | 1 | 1 | 0.036 |
| Ethion (OP) | 2 | 1 | 0.039 |
| Chlorpyrifos (OP) | 7 | 2 | 0.246 |
| Triazophos (OTP) | 3 | 0 | 0.057 |
| Profenofos (OP) | 3 | 0 | 0.071 |
OC: Organochlorine; OP: Organophosphate; OTP: Organothiophosphate, DDT: Dichloro-diphenyl-trichloroethane.
ELISA titres
The results of anti-Dsg-1 and Dsg-3 antibody titres have been represented in Figure 1. Figure 2 shows the distribution of anti-M3 Ach receptor antibodies. Table 2 shows the titres in the subgroups of husbands and wives and those with detectable pesticide blood levels. Anti-Dsg3 antibody levels were significantly higher in the husbands (p=0.029), although it was much less than the cut-off values used for diagnosis of clinical disease (20 RU/mL). The mean titres of the other two markers were statistically similar in both husbands and wives. There was no statistically significant association between the presence of pesticide in sera and anti-Dsg-1, anti-Dsg-3, and anti-M3 Ach receptor antibody titres.
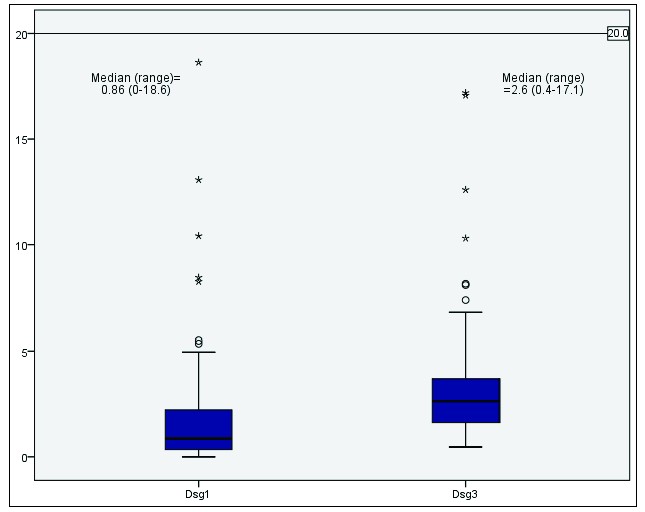
- Box plot showing the distribution of anti-Dsg-1 and 3 antibody titres.
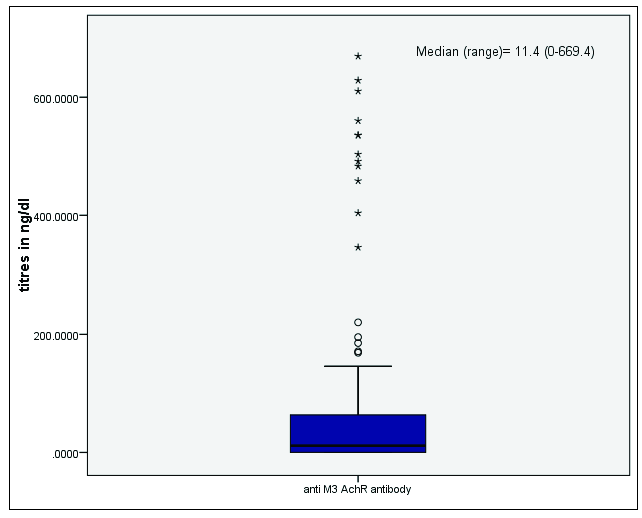
- Box plot showing median and range of anti-M3 AChR antibody titres.
| Anti desmoglein 1 antibody ELISA (RU/mL) | Anti desmoglein 3 antibody ELISA (RU/mL) | Anti M3 acetyl choline receptor antibody ELISA (ng/dL) | ||
|---|---|---|---|---|
| Husbands | Median (IQR) | 1.3 (0.52 to 2.46) | 3.22 (1.62 to 4.82) | 0.40 (0 to 115.5) |
| Wives | Median (IQR) | 0.63 (0.26 to 1.98) | 2.52 (1.53 to 3.35) | 16.88 (0 to 56.05) |
| P value | 0.188 | 0.029 | 0.818 | |
| With detectable serum pesticides | Median (IQR) | 0.96 (0.42-2.6) | 3.13 (2.21-4.45) | 16.88 (0- 83.02) |
| With undetectable serum pesticides | Median (IQR) | 0.79 (0.25-1.74) | 2.58 (1.35-3.59) | 2.91 (0- 84.09) |
| P value | 0.92 | 0.15 | 0.59 | |
ELISA: Enzyme-linked immunosorbent assay; IQR: Interquartile range. Bold font signifies significant p value.
Correlations
No significant correlation was found between the duration of pesticide exposure as represented by PEM and anti-Dsg-1 (r=0.17, p=0.11), anti-Dsg-3 (r=0.19, p=0.07), or anti-M3 ACh receptor antibody titres (r=-0.03, p=0.74). Anti-Dsg-1 antibodies had a significant positive correlation with serum levels of chlorpyrifos (r=0.3, p=0.005). Blood levels of none of the other individual pesticides had any correlation with any of the antibody titres.
Discussion
Environmental factors, in addition to genetic predisposition, play an important role in the pathogenetic mechanisms behind pemphigus. In a study from Iran, Valikhani et al.7 found that 14.8% of pemphigus patients reported exposure to pesticides which was three times higher than healthy controls. In another study from Israel, 23% of pemphigus patients were exposed to chemicals, including pesticides.8 In a multicentre study to assess the association between pemphigus and environmental agents, exposure to pesticides was the highest among pemphigus patients from Bulgaria (39%), followed by Israel (21.9%).9 The above-mentioned studies did not elaborate on the degree of pesticide exposure, nor did they quantify pesticide blood levels. Exposure to several pesticides, especially organophosphates, has been linked to contact pemphigus. These include pemphigus vulgaris after exposure to diazinon (OP)10, DDT11, chlorpyrifos12, phenol13, paraquat14, and glyphosate15. In a recent study from Brazil, pesticide levels in hair were measured in individuals with pemphigus vulgaris, pemphigus foliaceous, and healthy controls. All three groups had pesticide residues in hair, with it being more frequently detected in pemphigus foliaceous compared to vulgaris. Pemphigus vulgaris patients did not test positive for OP compounds.16
In our study, pesticides were detected in the sera of both husbands and wives. Interestingly β- endosulfan was detected among the females, even though they did not report any occupational exposure to pesticides. Endosulfan is a ubiquitous environmental contaminant that can travel long distances due to its semi-volatile nature.4 This could suggest that non-occupational environmental exposure to pesticides could also be present. Even though a few Indian states like Kerala and Karnataka have banned the use of endosulfan, India is one of its largest producer and consumer.4 Chlorpyrifos was the most common pesticide detected among husbands. It is an organophosphate compound and has been reported to cause contact pemphigus. Further, a study was done using the sera of two patients with contact pemphigus caused due to chlorpyrifos. Normal human skin specimens were incubated with serial dilutions of chlorpyrifos and served as substrates for indirect immunofluorescence (IIF) for sera from the pemphigus patients. They were compared with substrates with normal media. An interferon γ release assay was also done in the presence of chlorpyrifos. It was seen that IIF was more strongly positive in the specimen incubated with chlorpyrifos. Interferon γ assay was also positive in both pemphigus patients. The authors suggested that this method of IIF and IFN γ cytokine assay may serve as an in vitro diagnostic test for suspected pesticide-induced pemphigus.12
Pesticides are known to interfere with the growth and survival of leukocytes by inducing apoptosis or cell cycle arrest and affect the specific immunological functions of Natural Killer cells, T cells, B cells, and macrophages.17 The association between pesticide exposure and the development of autoimmunity has been studied. In a study, the anti-nuclear antibodies were measured in agricultural farmers. It was found that although the overall duration of pesticide exposure did not correlate with the anti-nuclear antibody titres; moderate to high titres were associated with the use of specific pesticides like heptachlor (organochlorine) and diazinon (organophosphate).18
In a study by Torzecka et al.19, circulating anti-Dsg-1/3 antibodies were detected by ELISA in 19.4% of healthy first-degree relatives of pemphigus patients. This was significantly greater than the healthy controls, suggesting that individuals who are genetically related to pemphigus patients might have significant levels of circulating antibodies, but not have clinical disease.20
The role of environmental factors in the development of positive anti-Dsg-1 and anti-Dsg-3 antibodies has been studied in endemic pemphigus foliaceous. These antibodies have been assessed in sera of healthy individuals residing in the endemic region of Peru. It was found that 47.6% of individuals had positive antibody titres suggesting that they could have been exposed to environmental factors like haematophagous insects like black flies, exposure to cereal dust and fumes responsible for triggering pemphigus.21
In our study, none of the participants had anti-Dsg-1/ Dsg-3 titres higher than the 20 RU/mL cut-off. Although we had included wives as the control population (not directly exposed to pesticides), the frequency of detection of pesticides in sera and antibody titres in them did not differ significantly from the husbands. In addition, the antibody titres did not correlate with PEM.
Anti-ACh receptor antibodies have been found in pemphigus patients. In a study from India, it was seen that anti-M3 ACh receptor antibodies correlated with disease severity and anti-Dsg-1 antibody.22 Whether these antibodies have a direct pathogenic role or are just an epiphenomenon remains to be ascertained.23 We had chosen M3ACh receptor antibodies as part of our study because organophosphate pesticides have been thought to trigger pemphigus through mechanisms involving muscarinic ACh receptors. We found similar titres in both husbands and wives. It did not show any significant correlation with PEM either.
Limitations
Considering the rarity of clinically manifest pemphigus, a large number of healthy subjects need to be studied in the current study design. They may also be followed in time to detect incident pemphigus. Alternatively, the expression of adhesion proteins or their adhesion function can be assessed in keratinocyte cell lines upon incubation with varying concentrations of pesticides. Pesticide levels can also be assessed in diagnosed pemphigus patients, and the levels can be correlated with antibody titres and disease severity to establish an association between pesticides and pemphigus. Determining these aspects is cost-intensive and was not done in the current research. These form subjects for future research.
Conclusion
Anti-Dsg-3 titres were significantly higher in husbands, compared with wives. Anti-Dsg-1 titres had a significant positive correlation with chlorphyriphos serum levels. The biomarkers of pemphigus, namely anti-Dsg-1 and anti-Dsg-3 did not increase to diagnostic levels (>20 U/ mL) in any of the healthy participants exposed to pesticides.
Ethical approval
The research/study was approved by the Institutional Review Board at the Postgraduate Institute of Medical Education and Research, Chandigarh, number PGI/IEC/2018/002011, dated 12/12/2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The study was funded by the Indian Association of Dermatologists, Venereologists and Leprologists Research Grant- 2018
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Pemphigus: Etiology, pathogenesis, and inducing or triggering factors: Facts and controversies. Clin Dermatol. 2013;31:374-81.
- [CrossRef] [PubMed] [Google Scholar]
- Review on pathogenesis of pemphigus. Hong Kong Dermatology Venereol Bull. 2002;10:62-8.
- [Google Scholar]
- A retrospective study of antihypertensives in pemphigus: A still unchartered odyssey particularly between thiols, amides and phenols. Arch Med Sci. 2015;11:1021-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip Toxicol. 2009;2:1-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pesticide metabolite and oxidative stress in male farmers exposed to pesticide. Ann Occup Environ Med. 2017;29:5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pemphigus and associated environmental factors: A case-control study. Clin Exp Dermatol. 2007;32:256-60.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus in Israel-an epidemiologic analysis of cases in search of risk factors. Isr Med Assoc J.. 2003;5:410-2.
- [PubMed] [Google Scholar]
- Pemphigus vulgaris: Environmental factors occupational, behavioral, medical, and qualitative food frequency questionnaire. Int J Dermatol. 2001;40:562-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus vulgaris induced by diazinon and sun exposure. Dermatology. 2000;201:378-9.
- [CrossRef] [PubMed] [Google Scholar]
- Contact pemphigus induced by dihydrodiphenyltrichlorethane. Eur J Dermatol. 1998;8:442-3.
- [PubMed] [Google Scholar]
- Chlorpyrifos exacerbating pemphigus vulgaris: A preliminary report and suggested in vitro immunologic evaluation model. Skinmed. 2006;5:111-3.
- [CrossRef] [PubMed] [Google Scholar]
- A case of phenol-related contact pemphigus. Dermatology. 2001;203:355-6.
- [CrossRef] [PubMed] [Google Scholar]
- Case report: transdermal absorption of paraquat-induced poisoning combined with pemphigus vulgaris. Biomed Res.. 2017;28:4.
- [Google Scholar]
- Measurement of pesticides in hair samples from pemphigus foliaceus and pemphigus vulgaris patients in Southeastern Brazil. An Bras Dermatol. 2023;98:644-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adverse effects of pesticides on the functions of immune system. Comp Biochem Physiol C Toxicol Pharmacol. 2020;235:108789.
- [CrossRef] [PubMed] [Google Scholar]
- Lifetime pesticide use and antinuclear antibodies in male farmers from the agricultural health study. Front Immunol. 2019;10:1476.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circulating pemphigus autoantibodies in healthy relatives of pemphigus patients: Coincidental phenomenon with a risk of disease development? Arch Dermatol Res. 2007;299:239-43.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating pemphigus igG in families of patients with pemphigus: Comparison of indirect immunofluorescence, direct immunofluorescence, and immunoblotting. J Am Acad Dermatol. 1997;36:44-52.
- [CrossRef] [PubMed] [Google Scholar]
- Antidesmoglein 1 and 3 antibodies in healthy subjects of a population in the peruvian high amazon. Int J Dermatol. 2018;57:344-48.
- [CrossRef] [PubMed] [Google Scholar]
- A hospital-based epidemiological study of corrosive alimentary injuries with particular reference to the Indian experience. Natl Med J India. 2013;26:31-6.
- [PubMed] [Google Scholar]
- Correlation of IgG autoantibodies against acetylcholine receptors and desmogleins in patients with pemphigus treated with steroid sparing agents or rituximab. PLoS One. 2020;15:e0233957.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







