Translate this page into:
Concurrent pyoderma gangrenosum and tuberculosis: Diagnosis, treatment complexities and exploring cyclosporine’s role – A literature review
Corresponding author: Dr. Surabhi Sinha, Department of Dermatology, Atal Bihari Vajpayee Institute of Medical Sciences and Dr. Ram Manohar Lohia Hospital (ABVIMS and RML Hospital), Baba Kharak Singh Marg, Connaught Place, New Delhi, India. surabhi2310@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dhir B, Sinha S, Sardana K, Goutam P, Ahuja A. Concurrent pyoderma gangrenosum and tuberculosis: Diagnosis, treatment complexities and exploring cyclosporine’s role – A literature review. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_637_2023
Dear Editor,
Pyoderma gangrenosum (PG) is a chronic, progressive ulcerative disease often associated with systemic conditions (50–70%). Recently, the association of PG with tuberculosis (TB) has been increasingly reported.1–6 Here, we report a diabetic male with PG and TB and explore the complex relationship between PG and TB.
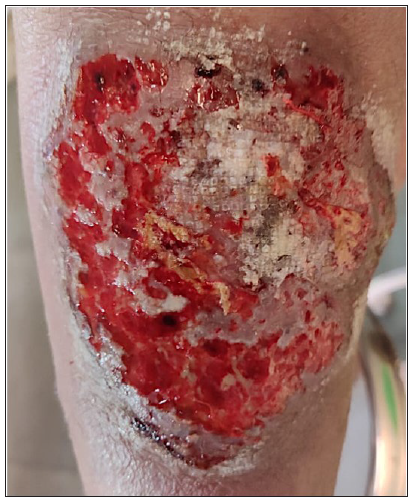
A 48-year-old diabetic male presented with painful, expanding ulcers on his legs for 2 months. Four months earlier, the patient experienced low-grade fever, abdominal pain, and gradual weight loss. Triple-phase computed tomography revealed multiple, hypodense and variable-sized parenchymal lesions in the spleen and liver. Biopsy from the hepatic lesions revealed multiple ill-defined epithelioid cell granulomas with Langhans giant cells. The patient did not have a history of altered bowel habits, loose stools, or melena. In addition, stool tests for occult blood and fecal calprotectin were negative, whereas Mantoux and Interferon Gamma Release Assay (IGRA) were positive leading to the exclusion of inflammatory bowel disease (Crohn’s). Two months into antitubercular therapy (ATT), ulcerative lesions developed on the legs. The patient presented with large, painful ulcers with perilesional erythema and tenderness [Figure 1].

- Giant ulcer at a presentation on right leg.
The patient was evaluated, and the summary of significant findings are summarised in Table 1. There was no organism isolated in serial pus cultures. Histopathology from the ulcer edge [Figure 2] revealed pan-dermal perivascular mixed infiltrate with endothelial swelling and neutrophilic infiltrate of the wall but no fibrinoid necrosis. Based on these features, the patient was diagnosed as PG.
| Investigations | Value |
|---|---|
| TLC † (109/L) | 7,600 |
| Platelet count (109/L) | 150 |
| Kidney and Liver function | Within normal limits |
| ANA* | +; Line immunoassay –ve |
| p-ANCA, c-ANCA § | Negative |
| CRP (mg/L) ¶ | >0.6 |
| RA Factor †† | + |
| Ultrasonography (Abdomen) | Hepatomegaly with grade I fatty liver. Hepatic hypoechoic lesions with central anechoic areas in segment VIll/VIl with similar lesions in splenic parenchyma. |
| Pus culture | No growth in the three serial cultures |
| Pathergy | Negative |
| Stool for occult blood | Negative |
| Triple-phase computed tomography (Chest and abdomen) | Multiple hypodense splenic parenchymal lesions with no significant enhancement, larger one measuring ∼27 × 20 mm towards the upper pole region. A few similar lesions are also seen in segments VIlI and VIl of hepatic parenchyma. Findings suggestive of granulomatous aetiology |
| Serum cyclosporine (2 h) | 260 ug/L |
| AFP, CEA, CA 19-9 ** | Negative |
† TLC – Total leucocyte count, *ANA – Antinuclear antibody, § p-ANCA, c-ANCA - Antineutrophilic cytoplasmic antibody, ¶ CRP – C-reactive protein, †† RA – Rheumatoid factor, **AFP – Alpha Feto Protein, CEA – Carcinoembryonic Antigen, CA 19-9 – Carbohydrate antigen 19-9

- Histopathological examination from ulcer edge: Shows hyperkeratosis with irregular acanthosis. Dermis shows pan-dermal perivascular and peri-adnexal mixed inflammatory infiltrate. (Haematoxylin & Eosin, 40x)
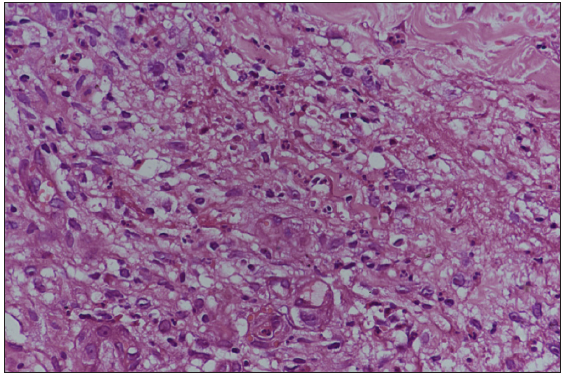
- Mixed inflammatory infiltrate comprising lymphocytes, plasma cells, neutrophils, and eosinophils. Few vessels showed endothelial swelling with neutrophilic infiltrate of the wall but no fibrinoid necrosis. (Haematoxylin & Eosin, 400x)
Managing the condition posed challenges as concurrent TB and poorly controlled diabetes limited the use of systemic steroids and other immunosuppressants. Previously well-controlled diabetes became erratic upon admission (Blood sugar >200 mg/dL). Local wound care was complicated by active pathergy, managed with placental extract and non-adhesive dressings. Cyclosporine was initiated at 3 mg/kg, later increased to 5 mg/kg due to inadequate response, due to the CYP3A4 enzyme-inducing effects of Rifampicin. Cyclosporine levels 2 h after the first dose reached 260 µg/L which was deemed adequate. A combination of oral cyclosporine and careful local wound care yielded substantial results at 4 weeks [Figure 3]. The dose of cyclosporine was reduced after 6 weeks and completely stopped after 4 months. The patient is currently in remission and has completed 6 months of ATT.
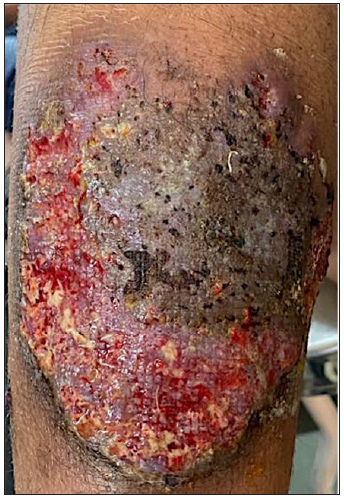
- Ulcer at day 1 of cyclosporine treatment.
- Healing of ulcer on right leg on day 14 of starting cyclosporine.
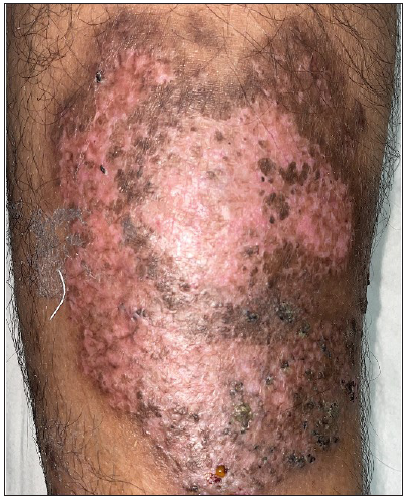
- Healing of ulcer on right leg at day 90.
Approximately, 50–70% of PG patients exhibit underlying systemic diseases, preceding or following PG, including inflammatory bowel disease, monoclonal gammopathy, and malignancy. Treatment of the associated systemic disease can lead to partial or complete resolution.
The association of PG and TB has been perplexing. On one hand, there are reports of latent as well as active TB being associated with the onset of PG. On the other hand, immunosuppressive therapy for PG may lead to the activation of TB. There are reports of PG healing in the treatment of latent TB. Multiple case reports document the coexistence of TB and PG, with various presentations of TB, including active and latent forms, and a reported TB reactivation during PG treatment.1–6 Table 2 presents a summary of all available case reports on the coexistence of PG and TB. In most instances of active TB cases with comorbid conditions, patients received ATT alongside immunosuppressive/biologic therapy, except for one case where the ulcer was re-epithelialized solely on ATT.4
| S.No. | Authors/Year | Type of tuberculosis | Comorbidities | Treatment and outcome of PG |
|---|---|---|---|---|
| 1. | Saklecha et al.1 | 14 years with Latent TB | None | INH, 300 mg/day; new lesions of PG stopped after 3 months of Isoniazid Patient disease-free after 1 year of stopping INH (9-month course) |
| 2. | Haghighi et al.2 | 49 years with Lymph Node Tuberculosis | None | Prednisone and cyclosporine |
| 3. | Patvekar et al.3 | 49 years with bone TB | Ulcerative colitis | Dexamethasone – cyclophosphamide pulse and ATT |
| 4. | Zaraa et al.4 | 60 years with LN TB | None | None; resolved with ATT |
| 5. | Romańska-Gocka et al.5 | 49 years with pulmonary Kochs; ulcers started after Mantoux test | Monoclonal IgA gammopathy | Prednisone, dapsone, cyclosporine A |
| 6. | Nanoudis et al.6 | 28 years with resistant TB | Chronic granulomatous disease, Aspergillus pneumonia | Dapsone, Intravenous immune globulin (IVIG), and intravenous pulse methylprednisolone 1g/day for 3 days f/b tab Methylprednisolone 16 mg; healed after 3 months |
INH – Isoniazid, LN – Lymph Node
Our patient developed lesions 2 months after starting ATT, worsening continuously, raising concerns about ATT as a triggering factor. Despite an extensive review, no previous reports linked ATT drugs to PG. The Naranjo adverse drug reaction probability scale score of 1, along with the patient’s improvement after restarting ATT (discontinued for 3 weeks), essentially ruled out drug-induced PG. TB is known to trigger system-wide inflammation which could potentially lead to PG, explaining the increasing number of reports of the coexistence of PG and TB.
Poorly controlled diabetes and TB prompted us to select cyclosporine over systemic corticosteroids, despite the lack of a completely ‘safe’ treatment option. Oral cyclosporine, an alternative first-line treatment for patients with contraindications to glucocorticoid therapy, has demonstrated comparable efficacy to prednisolone in randomised controlled trials (RCT).7 However, due to the effects of rifampicin and pyrazinamide on serum levels, the dose of cyclosporine needed to be adjusted and monitored accordingly. The use of immunosuppressants in TB requires careful consideration with none deemed entirely safe. Decisions should be individualised due to potential complications, emphasising the importance of vigilance for drug interactions, particularly with anti-TB medications such as rifampicin.
Managing PG becomes complex when combined with TB and diabetes. This case demonstrates significant improvement with oral cyclosporine and local wound care, emphasising the crucial need for careful management in the presence of concurrent systemic diseases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Resolution of chronic recurrent pyoderma gangrenosum with latent tuberculosis treatment. Pediatr Dermatol. 2021;38:181-3.
- [CrossRef] [PubMed] [Google Scholar]
- Lymph node tuberculosis associated with pyoderma gangrenosum. Infect Med. 2005;22:76-9.
- [Google Scholar]
- Pyoderma gangrenosum with an underlying ulcerative colitis associated with bone tuberculosis. Indian Dermatol Online J. 2013;4:43.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pyoderma Gangrenosum and lymph nodes tuberculosis disease: Unusual association. Dermatol Rep. 2011;3:e8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pyoderma gangrenosum with monoclonal IgA gammopathy and pulmonary tuberculosis. Illustrative case and review. Adv Dermatol Allergol. 2015;2:137-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pyoderma gangrenosum in a patient with chronic granulomatous disease. Med (United States). 2017;96(31)
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparison of the two most commonly used treatments for pyoderma gangrenosum: Results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





