Translate this page into:
Congenital asymptomatic papule on the lower eyelid
Correspondence Address:
Arun C Inamadar
Department of Dermatology, Venereology and Leprosy, SBMP Medical College, Hospital and Research Center, BLDE University, Bijapur - 586 103, Karnataka
India
| How to cite this article: Adya KA, Palit A, Inamadar AC. Congenital asymptomatic papule on the lower eyelid. Indian J Dermatol Venereol Leprol 2018;84:578-580 |
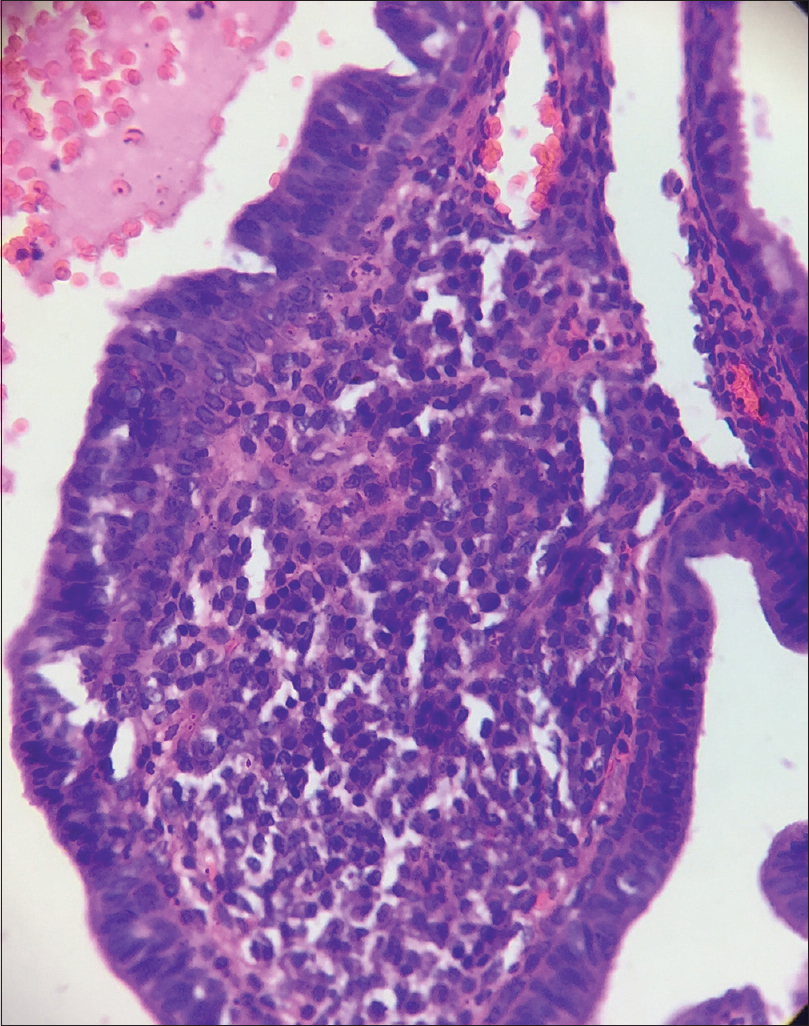
A 10-year-old boy presented with a congenital asymptomatic gradually enlarging, skin-colored, circumscribed papule measuring about 3 mm × 3 mm with central umbilication and superficial scaling, on the lateral aspect of the left lower eyelid [Figure - 1]. Dermoscopy showed a circumscribed lesion with perilesional erythema, surface scaling and a hyperpigmented core embedded in the center of the lesion [Figure - 2]. Histopathological analysis of the lesion revealed papillary structures invaginating into the dermis, composed of a fibrovascular core of mononuclear cells, lined by cuboidal to low columnar cells. At the surface, this lining epithelium was continuous with the epidermis [Figure - 3], [Figure - 4], [Figure - 5].
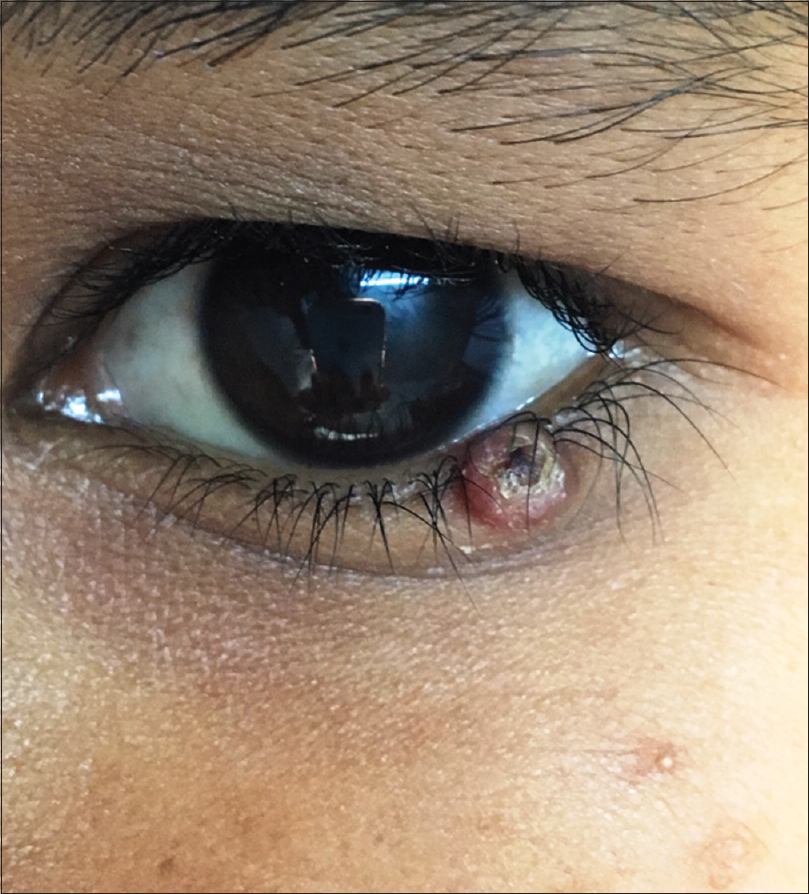 |
| Figure 1: A 3 mm × 3 mm circumscribed papule with central umbilication on the left lower eyelid |
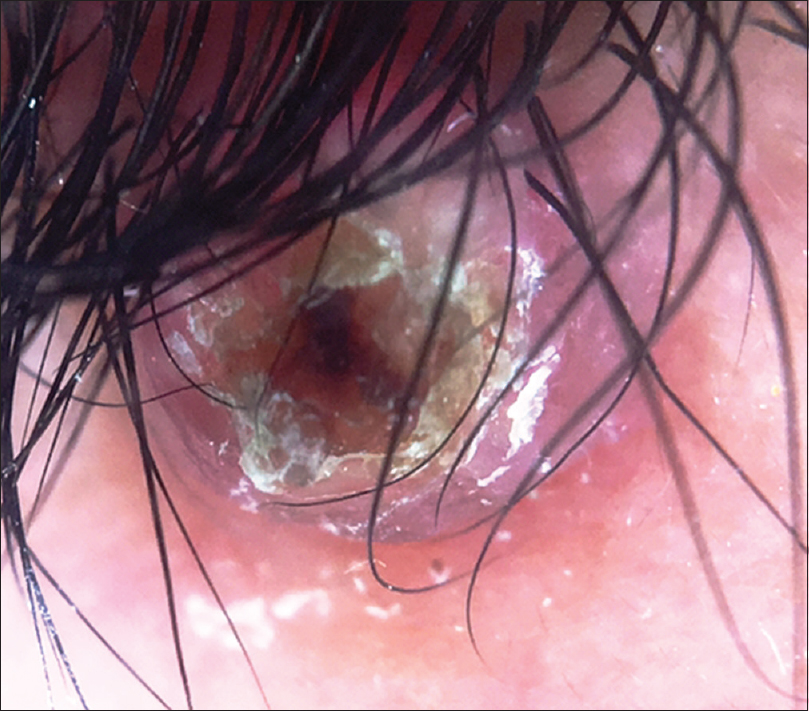 |
| Figure 2: Noncontact dermoscopy under polarized light using DermLite-DL3™ revealed a circumscribed papule with surface scaling surrounded by erythema and a hyperpigmented core embedded in the center of the lesion (×10) |
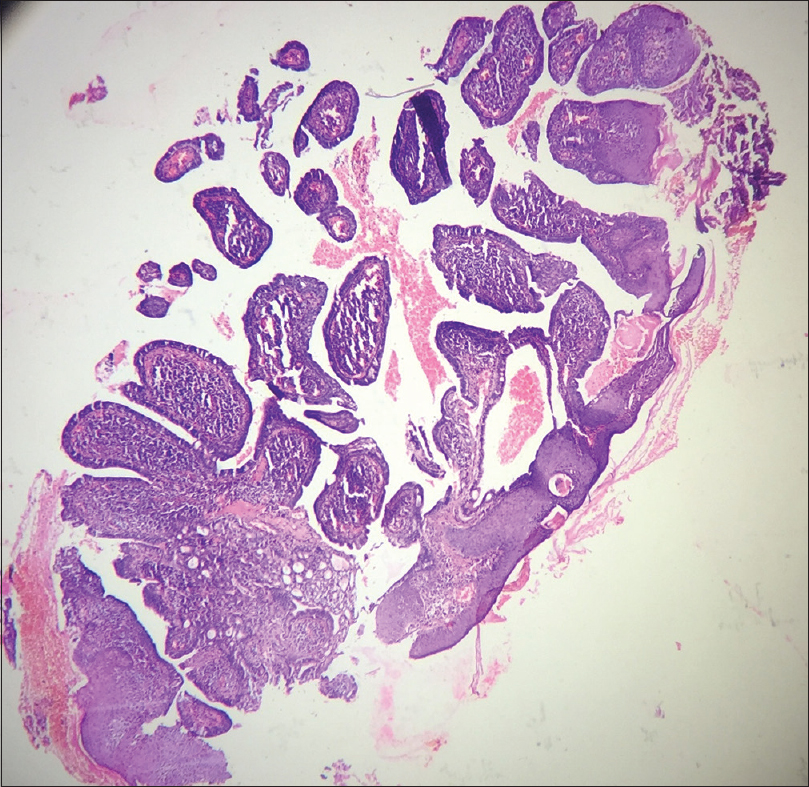 |
| Figure 3: Scanner view photomicrograph of the lesion shows papillary invaginations into the dermis, continuous with the epidermis (H and E, ×50) |
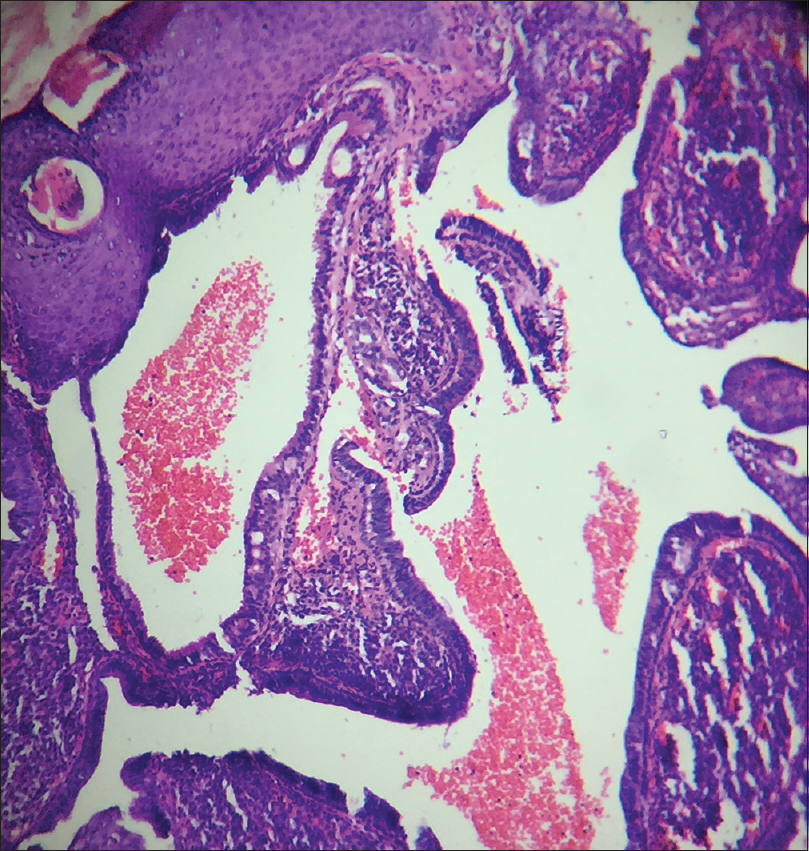 |
| Figure 4: Papillary invaginations into the dermis showing a fibrovascular core lined by cuboidal or low columnar cells which continue as epidermis at the surface (H and E, ×100) |
 |
| Figure 5: Fibrovascular cores of the invaginations composed of connective tissue and interspersed blood vessels (H and E, ×400) |
Question
What is your diagnosis?
Answer
Syringocystadenoma papilliferum.
Discussion
Syringocystadenoma papilliferum is considered a hamartoma of the eccrine/apocrine glands, usually appearing as a solitary, well-circumscribed, dome-shaped pinkish nodule. It can be present at birth in 50% of the cases, predominantly involving the head and neck region. Origin from a preexisting nevus sebaceous is also not uncommon. Multiple lesions configured linearly or in groups may be seen as well. Many of the lesions may show a central umbilication or vesicle-like inclusion containing clear fluid. Histopathologically, the lesions demonstrate papillary invaginations into the dermis, composed of a fibrovascular core lined by cuboidal or low columnar epithelium showing apical decapitation snouts at places. At the surface, this epithelium continues as epidermis with a zone of transition. Syringocystadenoma papilliferum is a benign condition and is managed successfully by simple excision. Malignant transformation is rare, which is evidenced by rapid enlargement, bleeding or ulceration.[1],[2],[3]
Differential diagnoses for such a presentation include molluscum contagiosum, trichofolliculoma and apocrine hidrocystoma. Although molluscum contagiosum possesses a central umbilication and can be fleshy, it is an acquired condition. Trichofolliculoma may appear quite similar, but the central umbilication is composed of a tuft of vellus hair.[4] Apocrine hidrocystoma is mostly periocular in location but frequently occurs near the outer canthus and has a cystic appearance without central umbilication.[5] Some of the histopathological differentials include tubular apocrine adenoma, hidradenoma papilliferum and metastatic adenocarcinoma. Tubular apocrine adenoma shows lobules of well-differentiated tubular structures of apocrine differentiation situated in the dermis. The fibrous tissue stroma has only sparse inflammatory cells as opposed to syringocystadenoma papilliferum which shows a prominent inflammatory infiltrate, especially of plasma cells. Hidradenoma papilliferum although almost always occurs in the vulval and perianal regions, the involvement of eyelid has also been documented. Histopathologically, the tumor is located in the dermis and shows both papillary and glandular structures, usually lined by tall columnar cells with pale eosinophilic cytoplasm and umbilication at their apices. The papillae have an arborizing trabecular morphology.[6] A metastatic adenocarcinoma usually demonstrates irregular glands infiltrating a fibrous stroma predominantly in reticular dermis, showing architectural irregularity and cellular atypia.[7]
As all these entities are of glandular origin and share a common histopathological feature of papillary structures in the dermis, immunohistochemistry will be helpful in differentiating them from one another when the site of involvement is the same. The columnar cells in syringocystadenoma papilliferum express gross cystic disease fluid protein 15, carcinoembryonic antigen and cytokeratins 7 and 19, whereas the basal cuboidal cells express cytokeratins 5, 7, 8 and 14.[8] Tubular apocrine adenoma shows positivity for carcinoembryonic antigen and S-100.[9] Gross cystic disease fluid protein 15 is a sensitive marker for apocrine differentiation which is usually positive in hidradenoma papilliferum.[6] Caudal-related homeobo ×2, cytokeratins 7 and 20, thyroid transcription factor 1, carcinoembryonic antigen, MUC2, MUC5AC, SMAD4, estrogen receptor and gross cystic disease fluid protein 15 are employed for metastatic adenocarcinoma as immunohistochemical markers according to the site of the primary tumor.[10]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Farrant P, Mowbray M, Sinclair RD. Dermatoses of the scalp. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. Oxford: Wiley-Blackwell; 2010. p. 107.1-107.15.
[Google Scholar]
|
| 2. |
James WD, Berger TG, Elston DM, Neuhaus IM. Epidermal nevi, neoplasms and cysts. In: James WD, Berger TG, Elston DM, Neuhaus IM, editors. Andrews' Diseases of the Skin Clinical Dermatology. 12th ed. Philadelphia: Elsevier Saunders; 2016. p. 625-79.
[Google Scholar]
|
| 3. |
Wick MR, Barnhill RL. Sweat gland tumors. In: Barnhill RL, Crowson AN, Magro CM, Pipekorn MW, editors. Dermatopathology. 3rd ed. New York: McGraw Hill Companies, Inc.; 2010. p. 725-65.
[Google Scholar]
|
| 4. |
Al-Ghadeer H, Edward DP. Congenital sebaceous trichofolliculoma of the upper eyelid. Ophthal Plast Reconstr Surg 2016. [Epub ahead of print]
[Google Scholar]
|
| 5. |
Pandit VS, Inamadar AC, Palit A, Sneha M. SkIndia Quiz 30: A cystic nodule in the periorbital region. Indian Dermatol Online J 2016;7:556-7.
[Google Scholar]
|
| 6. |
Weedon D. Tumors of cutaneous appendages. In: Weedon D, editor. Weedon's Skin Pathology. 3rd ed. Edinburgh: Churchill Livingstone, Elsevier; 2010. p. 758-807.
[Google Scholar]
|
| 7. |
Rabkin MS. Cutaneous metastases. In: Barnhill RL, Crowson AN, Magro CM, Pipekorn MW, editors. Dermatopathology. 3rd ed. New York: McGraw Hill Companies, Inc.; 2010. p. 960-79.
[Google Scholar]
|
| 8. |
Yamamoto O, Doi Y, Hamada T, Hisaoka M, Sasaguri Y. An immunohistochemical and ultrastructural study of syringocystadenoma papilliferum. Br J Dermatol 2002;147:936-45.
[Google Scholar]
|
| 9. |
Mittal S, Lynn DC, Downing KJ, Plackett TP. Tubular apocrine adenoma – Case report and systematic review. SAJ Case Rep 2015;2:203.
[Google Scholar]
|
| 10. |
Park SY, Kim BH, Kim JH, Lee S, Kang GH. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med 2007;131:1561-7.
[Google Scholar]
|
Fulltext Views
3,600
PDF downloads
1,955





