Translate this page into:
Contact allergy to topical corticosteroids and sunscreens
Correspondence Address:
Abir Saraswat
Indushree Skin Clinic, B-7, Indira Nagar, Lucknow 226016
India
| How to cite this article: Saraswat A. Contact allergy to topical corticosteroids and sunscreens. Indian J Dermatol Venereol Leprol 2012;78:552-559 |
Abstract
Topical corticosteroids and sunscreens are extensively used formulations, both as over-the-counter products and as prescription medicines. Topical corticosteroids are increasingly being recognized as causes of allergic contact dermatitis. Because of their anti-inflammatory property, contact allergy to these agents may be difficult to suspect and prove. With corticosteroid allergy, there are special issues in patch testing that need to be considered: Screening tests need to be done with budesonide and tixocortol pivalate, and delayed readings are essential to pick up all positive cases. Preventive advice needs to be tailored according to the structural and chemical peculiarities of a particular molecule. Sunscreen allergy is a significant part of cosmetic allergy; especially in cases of photoallergic reactions. Each passing decade is bringing forth new allergens in this class. In many countries, benzophenones have recently been replaced by octocrylene as the leading causes of contact dermatitis to sunscreens. This article provides a broad overview of corticosteroid and sunscreen allergy so that the readers are aware of these important emerging classes of allergens.Topical Corticosteroids
Topical corticosteroids (TCS) are some of the most frequently used agents in modern topical therapy. They exhibit anti-proliferative, immunosuppressive, and hormonal activities. Almost 6 decades after their introduction, [1] they continue to be used extensively both as over-the-counter as well as prescription drugs in treating a wide variety of inflammatory dermatoses and are available alone or in combination in an array of topical formulations. Their extensive use has revealed several adverse effects, e.g. atrophy, striae, telangiectasia, rosacea, infections, hypertrichosis, allergic reactions, and problems related to systemic absorption.
It appears paradoxical that agents with anti-inflammatory and immunosuppressive activity can produce immediate or delayed-type hypersensitivity; but in 1959, just 7 years after their introduction, Burkhardt [2] reported the first case of contact allergy to TCS. Since then, similar reports have been published with increasing frequency and with higher number of patients. [3],[4],[5] With the introduction of more and more corticosteroid molecules and drug-delivery systems in the pharmaceutical market, the potential for TCS-induced contact dermatitis has increased exponentially. In the last decade or so, it has become clear that TCS hypersensitivity is probably an "iceberg" disease, and increased awareness and testing can reveal more cases than are diagnosed currently.
Epidemiology
A recent study from South-East Asia has revealed a positivity rate of 3.29% amongst all patch-tested patients over a 10-year period. [6] In studies from Europe and USA, the incidence from patch test clinic patients has ranged from 0.5% [3] to 10.7%. [4] Going by recent literature, there appears to be a rising trend in patch test positivity figures. However, this is at least partially a reflection of increasing recognition of this entity by dermatologists leading to increased patch testing with topical corticosteroids. Moreover, extended corticosteroid batteries are increasingly being used, leading to higher yield of positive results. [4] There is no data on the exact prevalence of TCS-induced contact dermatitis from India. However, double- or triple- combination creams containing corticosteroids are very popular and easily available without a prescription in our country. This definitely results in a lot of exposure to these molecules in our population, making the situation ripe for contact allergy.
Clinical Features
Contact allergy to TCS is not easy to diagnose and a high index of suspicion is essential to pick it up early. [Table - 1] lists the common clinical settings, signs, and symptoms, which should lead a clinician to consider the possibility of contact dermatitis to TCS. Most cases do not present with spectacular features because TCS allergy usually produces the very features of the dermatosis that they are used to treat, viz. erythema, scaling or oozing, and variable pruritus. However, sudden acute aggravations of pre-existing eczema do sometimes occur, and a diagnosis of TCS allergy becomes easy in these cases. In general, to maximize the chances of catching contact allergy to TCS, all patients who have long-standing or recurrent dermatitis like atopic dermatitis, stasis dermatitis or hand eczemas should at some point be tested for corticosteroid allergy. [7]

A common clinical situation is the patient who presents with prominent local adverse effects of TCS as a result of stronger and stronger products being used in the face of a lack of response in an otherwise steroid-responsive dermatosis. Lesions are usually papulovesicles with variable erythema corresponding to the site of the application of the TCS product. This situation should prompt vigorous attempts to look for TCS contact allergy.
Acute weeping dermatitis with severe edema, [8] id-like eruptions, erythema multiforme-like lesions at distant sites [9] and angioedema [10] have all been reported in cases of TCS-induced contact dermatitis. The eruption is initially localized to the site of use, but dissemination can occur later. In various studies, most of the patients had underlying stasis dermatitis, [11] atopic dermatitis, or allergic/irritant hand dermatitis. [12]
Systemic contact dermatitis to TCS is also well-recognized and presents as urticaria, exanthematous rash or purpura in previously-sensitized patients after administration of oral or inhaled corticosteroids. [13] Broadly, contact dermatitis to TCS should be on the list of differentials in all patients who use them for long periods.
Pathogenesis
There has been a lot of progress in the recent years in the understanding of delayed hypersensitivity to corticosteroids. It has been shown that sensitization occurs not to the TCS themselves, but to their degradation products. Upon application, an epidermal esterase rapidly cleaves any ester attached to the C21 position of the cyclopentanophenantherene ring of the corticosteroid molecule. This results in the formation of a highly reactive product called a corticosteroid-glyoxal. This product is a potent hapten and specifically combines with several protein residues to form allergens. The most potent antigenic complex is formed when the corticosteroid-glyoxal combines with the guanidyl residue of the amino acid arginine, and the ability and strength of this bonding with arginine largely determines the allergenicity of a TCS molecule. [14]
Other variations in corticosteroid structure also affect its rate of degradation and hence its allergenicity. Halogenation at C6 and C9 stabilizes the TCS molecule and renders them less liable to degradation and possible sensitization. [15] Similarly, the presence of a hydrogen atom at C17 reduces allergenicity, whereas an oxygen atom at C11 increases the chances of sensitization. [16],[17] Recently, methylation at C16 has also been shown to be a major predictor of interaction with arginine, and therefore, allergenic potential. [18]
Testing for Corticosteroid Allergy
Patch testing in a patient suspected of TCS-induced contact dermatitis needs special care for proper interpretation of results. Allergy due to a topical TCS-containing product can either be due to excipients (e.g. preservatives, penetration enhancers), allergens in the packaging (e.g. nickel from the tube) or the active ingredient(s). For this reason, a standard screening battery should always be applied in addition to the corticosteroid allergens. The most important change from usual patch testing procedure is an additional reading done at day 7, which picks up many reactions, which are either negative or equivocal at 48 or 96 hour readings. [19] Positive reactions developing even later than day 7 have also been reported, exemplifying the importance of follow-up in these cases. [20] Other peculiar reactions that are seen when patch testing with corticosteroids and their interpretations are shown in [Table - 2].
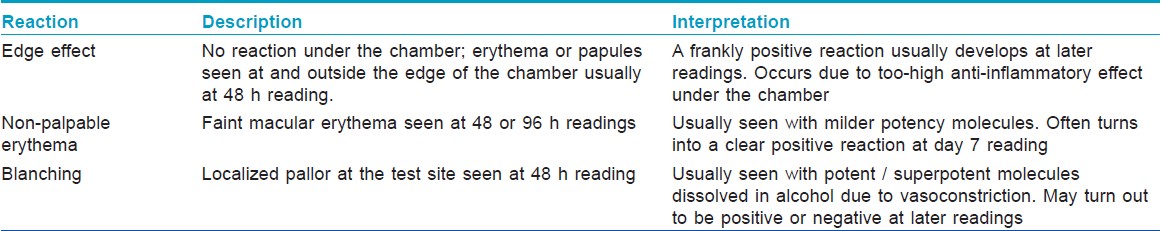
The current European Standard Series contains 2 corticosteroids budesonide 0.01% and tixocortol pivalate 0.1%, both in petrolatum. The Indian Standard Series approved by CODFI does not contain these allergens at present. However, they can easily be ordered from major suppliers. Most experts agree that these 2 are good screening agents for TCS allergy- [21] and are able to pick up a majority of cases of contact dermatitis to corticosteroids by virtue of their cross-reactivity patterns (vide infra). However, in a suspected case, a cream formulation of the suspected TCS product should always be added to these 2 screening agents as this significantly increases the diagnostic yield of the patch test. [22] In case of a negative result, patients with a strong clinical suspicion of steroid allergy should undergo further patch testing with an extended series containing all products ever used (as-is or as 1% ethanolic / petrolatum preparations). If the patient does not remember the products used, they should be tested with all products available in the market. As mentioned before, day 7 readings are mandatory.
Intradermal testing with corticosteroids has been shown to increase somewhat the diagnostic yield over patch testing, [23] but the high rate of local and distant adverse effects prevents its use outside specialist centers.
A repeated open application test (ROAT) done on the forearm is a good screening test for medicament allergy and can be used if a patient cannot undergo a patch test for any reason. However, it cannot delineate the exact allergen; therefore, effective preventive advice may not be possible in all cases. [24]
Patterns of Corticosteroid Cross Reactions
Dooms-Goossens et al[25] in 1986 reported several patients with positive patch test results with tixocortol pivalate when that molecule was not even available in that country. This occurred due to its structural similarity to hydrocortisone. This cross-reaction has been further confirmed in another study where all tixocortol pivalate-positive patients tested positive to intradermal reactions of hydrocortisone sodium phosphate although they were negative to patch testing with it. [26] Since then, tixocortol pivalate has been established as a sensitive marker for hydrocortisone, prednisolone, and methylprednisolone allergy. Similarly, budesonide cross-reacts with triamcinolone acetonide, hydrocortisone, hydrocortisone-17-butyrate, and prednicarbate and is a good proxy for them in patch testing.
Based on clinical data, statistical methods, and 3-dimensional molecular configuration studies, TCS have been divided into 4 groups form A to D [27] [Table - 3]. Cross-reactions usually occur between members of the same group, with important exceptions being the two screening molecules as noted above.

Treatment
Like all cases of contact dermatitis, successful treatment of TCS allergy is predicated on accurate identification of the culprit. After this has been achieved with careful patch testing and establishment of relevance, the patient should be explained the nature of the problem and should be provided a list of common brand names of all single-agent and combination products that contain the culprit molecule, as well as a list of possible cross-reactants. A replacement product should be prescribed. Ideally, it should not be a corticosteroid, and topical calcineurin inhibitors tacrolimus and pimecrolimus are perfect in this situation. If these do not give adequate relief or are contraindicated, an alternative TCS should be prescribed from a different group (vide supra) so that chances of a cross-reaction are minimized.
Conclusion
Contact allergy to TCS is often missed in the busy clinic owing to the unspectacular and non-specific nature of complaints in most cases. An alert physician can diagnose most cases if he maintains a high index of suspicion and does screening patch tests with tixocortol pivalate, budesonide, and the currently in-use product in suspected cases. Avoidance advice should strictly be based on the knowledge of cross reaction patterns in corticosteroid groups.
Sunscreens
Sunscreens are products that are placed in contact with human skin with the intention of absorbing, scattering, or reflecting solar UV radiation. The first "sun creams" were marketed in the 1930s in Europe and USA when sunbathing became fashionable. [28] Since then, many different chemicals have been introduced and used singly or in combination in various sunscreen products. With increasing awareness of the harmful effects of solar UV rays, sunscreen use is increasing rapidly all over the world. In addition to "pure" UV blocking products meant for daytime use on the skin, sun-screening chemicals are being incorporated in more and more different cosmetic products like moisturizers, body lotions, shower gels, shampoos, hair dyes, conditioners etc.
Sunscreens are expected to have multiple positive attributes: A high Sun Protection Factor (SPF), easy spreadability, high substantivity, sweat and water resistance, non-oily feel, and photostability. In addition, they have to be almost invisible upon application. These demands have led to the development of complex sunscreen products with very long ingredient lists in terms of both active agents and excipients, increasing their allergenic potential.
Epidemiology
Overall, sunscreens are a relatively minor part of allergic reactions caused by cosmetics. However, sunscreen use is increasing rapidly all over the world due to the media attention given to the carcinogenic and photoageing effects of solar radiation. In comparison, allergic reactions to these agents are still quite uncommon. The true incidence of sunscreen allergy is hard to estimate because many cases of photoallergic reactions are difficult to differentiate from the primary photosensitive dermatoses for which the sunscreen is being used. [29] Moreover, patch test concentrations of these agents may be too low owing to the fear of irritancy. [30] Lastly, the cumbersome photopatch test required to confirm cases of photoallergy is very infrequently performed outside of specialist centers. However, many recent reports from Europe and other western countries have highlighted increasing cases of allergic and photoallergic reactions to sunscreen products. [31],[32] Most reports of patients of photoallergic dermatitis or cosmetic allergy reveal close to 20% of them being attributed to sunscreen agents. [32],[33] There is no data on sunscreen allergy from India. However, sunscreen use in India is rapidly increasing, and there is no reason why allergic reactions to them should not occur here.
The epidemiologic profile of the typical patient with sunscreen allergy is that of an adult woman with a background of polymorphic light reaction or chronic actinic dermatitis. [32] This is most probably a reflection of increased sunscreen use by these groups of patients. Children are very rarely affected with contact allergy to sunscreens. Atopy and outdoor work are considered to be additional risk factors. [28]
Clinical Features
In a patient with a primary photosensitive dermatosis, a lack of expected response to treatment or a dramatic worsening after starting sunscreen use should prompt a search for a possible allergen in the sunscreen. In general, all cases of suspected cosmetic dermatitis, especially those that show photo-aggravation or exclusive photo-localization, should have sunscreen dermatitis in the differential diagnosis.
Sunscreen allergy most frequently manifests as dermatitis. Although this is usually subacute or chronic, an acute, vesicobullous dermatitis is not infrequent. [29] The severity of the reaction depends on the allergen, e.g. benzophenones are in general known to cause more severe reactions than cinnamates. [34] Most commonly, the reaction is photoallergic; i.e. the treated and sun-exposed parts are affected, whereas areas with sunscreen application that are unexposed to sun are spared. [35] Clinically, this is seen as sparing of upper eyelids or submental area or a sharp cutoff at the edge of the clothes. If the reaction is asymmetrical, it may be due to sunlight exposure from a window or due to connubial dermatitis caused by rubbed-off sunscreen from a close contact. Rarely, contact urticaria and anaphylaxis have been reported to benzophenones. [36] Erythema multiforme-like reaction has also been reported to this group of sunscreen agents. [37]
Allergens in Sunscreen Products
Mineral sunscreens like zinc oxide and titanium dioxide that reflect and scatter UV rays do not cause contact allergy. Sunscreen dermatitis is exclusively caused by the organic UV absorbing agents or by the excipients. Broadly, these agents are divided into the following categories:
UVB filters: In this category, para aminobenzoic acid (PABA) and its esters are the most common allergens. [38] PABA was one of the earliest sunscreens introduced in the market and it was soon realized that it and its esters glyceryl PABA and amyl dimethyl PABA had the potential to cause severe allergy; more commonly photoallergy. Only the ester octyl dimethyl PABA (Padimate-O® ) is nowadays in common use as it is considered to have a lower risk of causing photoallergy. However, PABA is still legal, and physicians should be aware that some brands of sunscreen or other cosmetic products may contain this agent. [39] PABA and its esters may cross-react with benzocaine and paraphenylene diamine due to their structural similarity.
Cinnamates are oil-soluble UVB filters that can cause photoallergic contact dermatitis. [40] These are very popular sunscreen ingredients by virtue of their water resistance and have been shown to be the culprits in 5% to 7% of all cases of photoallergic contact dermatitis in recent studies. [41],[42] Theoretically, they may cross react with food flavorings, fragrances, cinnamon extract, Balsam of Peru, etc., but recently, this concept has been questioned. [43]
Other UVB filters like salicylates, octyl triazone, and phenyl benzimidazole sulfonic acid are very infrequent allergens in spite of their widespread use.
UVA filters: Isopropyldibenzoylmethane was the first pure UVA filter, but it caused an epidemic of allergic and photoallergic dermatitis in Europe, which led to its withdrawal from the market in 1993. [44],[45] The only UVA filter in the market today is butylmethoxydibenzoylmethane (Avobenzone, Parsol 1789® ). It can cause photoallergy, but usually shows patch test positivity in combination with other sunscreen agents. [46]
Broad spectrum UV filters: Benzophenones are predominantly UVB filters, but their action spectrum extends into the UVA range as well. They are widely used in cosmetics, not only as sunscreens but also as preservatives to prevent formulations from photo-degradation. This group comprises 3 compounds, benzophenones-3,-4, and -10. Although all are very common sunscreen ingredients, benzophenone-3 or oxybenzone is the most popular. There was a mini-epidemic of benzophenone allergy in the 1990s in Europe. [47],[48] They have the propensity to cause severe reactions, [34] both immediate type viz. contact urticaria / anaphylaxis and delayed type viz. severe dermatitis and erythema multiforme-like reaction. Benzophenone-4 or sulisobenzone is considered a less common cause of photoallergy. This agent is commonly found in gel-based sunscreens, shower gels, and hair care products, and a recent report from England [49] suggests that sulisobenzone allergy may be more frequent than thought.
Octocrylene is a recently-introduced sunscreen agent that is chemically related to cinnamates. It has rapidly become very popular because it is cheap, photostable, has moisturizing and water resistant properties and it also photo-stabilizes cinnamates and butylmethoxydibenzoylmethane. It is used in most of the high SPF sunscreens available today. [1] In the last 8 years, increasing numbers of patients with allergy to octocrylene have been reported. [50] It is the only sunscreen agent that seems to cause contact allergy in young children not infrequently. [50] In adults, it usually causes photoallergy. It cross reacts with the NSAID ketoprofen, benzophenone-3, and other cinnamates. Therefore, it has been recommended that octocrylene-containing sunscreens should not be used in children and in patients who are allergic to ketoprofen or benzophenone-3. [28]
The newer broad spectrum UV filters such as drometrizole trisiloxane, methylene-bis-benzotriazolyl tetramethylbutylphenol (MBBT, Tinosorb M® ), and octyl triazone are only rare causes of contact dermatitis, with a few case reports each. [51],[52],[53]
Excipients: Sunscreens contain many excipients-- film-forming agents, surfactants, preservatives, fragrances, emulsifying agents, and moisturizers. All of these can cause contact allergy although it is distinctly uncommon compared to the active UV filters. In a study from Germany, [32] a high percentage of sunscreen-sensitive patients had positive patch and/or photopatch tests to perfumes and a lower percentage to preservatives.
Allergy Testing in Sunscreen Sensitivity
Sunscreen products may cause contact dermatitis as a result of allergy to the photo-protective agent(s), or less commonly to a vehicle ingredient. Patch testing should, therefore, be done with all ingredients. In patients with suspected cosmetic dermatitis or photoallergy, a screening series should be used to rule out allergy to other cosmetic ingredients like fragrances and preservatives. If negative, further testing should be done with a sunscreen series. With sunscreens, a photopatch test should always be done since they are very common photoallergens and many cases of true allergy will be missed if only patch testing is done. In photopatch testing, duplicate sets of allergens are applied and one of the sets is irradiated with 5 joules of UVA after 48 hours of occlusion. Interpretation of photopatch tests is presented in [Table - 4].
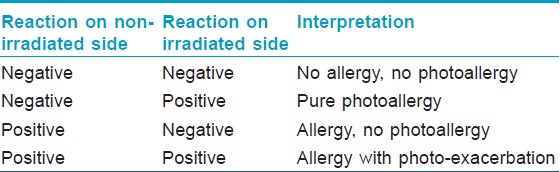
Sometimes, patients with sunscreen allergy may not react to any single sunscreen agent upon patch testing, but to specific combinations of products. This occurs by a phenomenon of co-allergy, and cases of combined photoallergy to benzophenones with cinnamates or with avobenzone have recently been reported. [54] Therefore, patients should always be tested with the suspected product as is, in addition to the specific battery. Once the allergen / photoallergen is identified, the patient should be told its name and preventive advice should be given in detail since many sunscreen ingredients are also present in other cosmetics.
On occasion, patch tests may be negative in spite of a strong clinical suspicion of allergy. In these cases, a Repeated Open Application Test (ROAT) on the forearm or a usage test on a previously-affected area may be performed to pick up contact allergy. The ROAT may need to be done for up to 2 to 3 weeks to develop positivity to some weak allergens.
If patch testing is not possible, then a simple way of treating sunscreen allergy is to advise the patient to use a product that contains only mineral sunscreens like zinc oxide or titanium dioxide. However, the author has come across a patient who had a photoallergic reaction to a sunscreen containing only zinc oxide. This patient was found to be allergic to parabens upon patch testing.
Conclusion
Sunscreen allergy is uncommon in spite of widespread and still increasing use. All patients with suspected cosmetic dermatitis or primary photosensitive disorders should be suspected of contact allergy to sunscreens. Common allergens in sunscreens vary according to their introduction in particular markets, and often small epidemics are reported. Currently, octocrylene is an increasingly-recognized allergen and with benzophenones is responsible for a majority of cases of sunscreen allergy reported in literature. Both patch and photopatch testing are essential to avoid false negatives in the diagnosis of sunscreen allergy. Excipient allergy, though less common than sunscreen agents, should also be kept in mind.
| 1. |
Sulzberger MB, Witten VH. The effect of topically applied compound F in selected dermatoses. J Invest Dermatol 1952;19:101-2.
[Google Scholar]
|
| 2. |
Burkhardt W. Kontaktekzem durch hydrocortisone. Hautarzt 1959;10:42-3.
[Google Scholar]
|
| 3. |
Alani MD, Alani SD, Allergic contact dermatitis to corticosteroids. Ann Allergy 1972;30:181-5.
[Google Scholar]
|
| 4. |
Davis MDP, el-Azhary RA, Farmer SA. Results of patch testing to a corticosteroid series; A retrospective review of 1188 patients during six years at Mayo clinic. J Am Acad Dermatol 2007;56:921-7.
[Google Scholar]
|
| 5. |
Isaksson M, Bruze M. Contact allergen of the year. Corticosteroids. Dermatitis 2005;16:3-5.
[Google Scholar]
|
| 6. |
Wattanakrai P, Temnithikul B, Pootongkam S. Pattern of corticosteroid allergy in Thailand. Dermatitis 2010;21:203-6.
[Google Scholar]
|
| 7. |
Browne F, Wilkinson SM. Effective prescribing in steroid allergy: Controversies and cross-reactions. Clin Dermatol 2011;29:287-94.
[Google Scholar]
|
| 8. |
Rodriguez-Serna M, Silvestre JF, Quecedo E, Martinez A, Miguel FJ, Gauchia R. Corticosteorid alleregy:report of 3 unusually acute cases. Contact Dermatitis 1996;35:361-2.
[Google Scholar]
|
| 9. |
Valsecchi R, Reseghetti A, Leghissa P, Cologni L, Cortinovis R. Erythema multiforme like lesions from triamcinolone acetonide. Contact Dermatitis 1998;38:362-3.
[Google Scholar]
|
| 10. |
Miranda-Romero A, Sanchez-Sambucety P, Bajo C, Martinez M, Garcia-Munoz M. Genital edema from contact allergy to prednicarbate. Contact Dermatitis 1998;38:228-9.
[Google Scholar]
|
| 11. |
Guin JD. Contact sensitivity to topical corticosteorids. J Am Acad Dermatol 1984;10:773-82.
[Google Scholar]
|
| 12. |
Alcantara- Villar M, Martinez Escribano J, Lopez Sanchez JD, Frias Iniesta J, Pagan Aliman JA. Corticosteroid induced contact dermatitis. Clinical management. Allergol Immunol Clin 1999;14:152-5.
[Google Scholar]
|
| 13. |
Mckenna DB, Murphy GM. Contact allergy to topical corticosteroids and systemic allergy to prednisolone. Contact Dermatiits 1998;38:121-2.
[Google Scholar]
|
| 14. |
Wilkinson SM, Jones SF. Corticosteroid usage and binding to arginine: Determinants of corticosteroid hypersensitivity. Br J Dermatol 1996;135:225-30.
[Google Scholar]
|
| 15. |
Lepoittevin JP, Drieghe J, Dooms-Goossens A. Studies in patients with corticosteorid allergy. Understanding cross-reactivity among different steroids. Arch Dermatol 1995;131:31-7.
[Google Scholar]
|
| 16. |
Fleischer D. Hydrocortisone. In: Connors KA, Amidon GL, Stella VJ, editors. Chemical stability of pharmaceuticals, a handbook for pharmacists. New York: John Wiley and Sons; 1986. p. 483-90.
[Google Scholar]
|
| 17. |
Wilkinson SM. Corticosteroid cross-reactions: An alternative view. Contact Dermatitis 2000;42:59-63.
[Google Scholar]
|
| 18. |
Baeck M, Chemelle J-A, Rasse C, el Terreux R, Goossens A. C16-methyl corticosteroids are far less allergenic than the non-methylated molecules. Contact Dermatitis 2011;64:1-8.
[Google Scholar]
|
| 19. |
Isaksson M, Bruze M, Goossens A, Lepoittevin JP. Patch testing with budesonide in serial dilutions: The significance of dose, occlusion time and reading time. Contact Dermatitis 1999;40:24-31.
[Google Scholar]
|
| 20. |
Subramanian S, Jerajani HA, Chowkekar S, Karkhanis A. Delayed patch test positivity to corticosteroids in chronic eczema. Contact Dermatitis 2006;54:227-8.
[Google Scholar]
|
| 21. |
Boffa MJ, Wilkinson SM, Beck MH. Screening for corticosteroid contact hypersensitivity. Contact Dermatitis 1995;33:149-51.
[Google Scholar]
|
| 22. |
Freeman S. Corticosteroid allergy. Contact Dermatitis 1995;33:240-2.
[Google Scholar]
|
| 23. |
Seukeran DC, Wilkinson SM, Beck MH. Patch testing to detect corticosteroid allergy: Is it adequate? Contact Dermatitis 1997;36:127-30.
[Google Scholar]
|
| 24. |
Wilkinson SM, English JS. Patch tests are poor detectors of corticosteroid allergy. Contact Dermatitis 1992;26:67-8.
[Google Scholar]
|
| 25. |
Dooms-Goossens A, Verschaeve H, DeGreef H, van Berendoncks J. Contact allergy to hydrocortisone and tixocortol pivalate: Problems in the detection of corticosteroid sensitivity. Contact Dermatitis 1986;14:94-102.
[Google Scholar]
|
| 26. |
Wilkinson SM, English JSC. Hydrocortisone sensitivity: A prospective study into the value of tixocortol pivalate and hydrocortisone acetate as patch test markers. Contact Dermatitis 1991;25:132-3.
[Google Scholar]
|
| 27. |
Coopman S, DeGreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol 1989;121:27-34.
[Google Scholar]
|
| 28. |
Avenel-Audran M. Sunscreen products: Finding the allergen. Eur J Dermatol 2010;21:161-6.
[Google Scholar]
|
| 29. |
Scheuer E, Warshaw E. Sunscreen allergy: A review of epidemiology, clinical characteristics, and responsible allergens. Dermatitis 2006;17:3-11.
[Google Scholar]
|
| 30. |
Goossens A. Contact allergic reactions to cosmetics. J Allergy (Cairo) 2011;2011:467-71.
[Google Scholar]
|
| 31. |
Berne B, Ros AM. 7 years experience of photopatch testing with sunscreen allergens in Sweden. Contact Dermatitis 1998;38:61- 4.
[Google Scholar]
|
| 32. |
Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. Review of a 15-year experience and of the literature. Contact Dermatitis 1997;37:221-32.
[Google Scholar]
|
| 33. |
Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol 2010;62:605-10.
[Google Scholar]
|
| 34. |
Landers M, Law S, Storrs FJ. Contact urticaria, allergic contact dermatitis, and photoallergic contact dermatitis from oxybenzone. Am J Contact Dermat 2003;14:33-4.
[Google Scholar]
|
| 35. |
Goossens A. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed 2004;20:121-5.
[Google Scholar]
|
| 36. |
Spijker GT, Schuttelaar ML, Barkema L, Velders A, Coenraads PJ. Anaphylaxis caused by topical application of a sunscreen containing benzophenone-3. Contact Dermatitis 2008;59:248-9.
[Google Scholar]
|
| 37. |
Zhang XM, Nakagawa M, Kawai K, Kawai K. Erythema-multiforme-like eruption following photoallergic contact dermatitis from oxybenzone. Contact Dermatitis 1998;38:43-4.
[Google Scholar]
|
| 38. |
Fisher AA. Sunscreen dermatitis: Para-aminobenzoic acid and its derivatives. Cutis 1992;50:190-2.
[Google Scholar]
|
| 39. |
Waters AJ, Sandhu DR, Lowe G, Ferguson J. Photocontact allergy to PABA in sunscreens: The need for continued vigilance. Contact Dermatitis 2009;60:172-3.
[Google Scholar]
|
| 40. |
Rodriguez E, Valbuena MC, Rey M, Porras De Quintana L. Causal agents of photoallergic contact dermatitis diagnosed in the National Institute of Dermatology of Colombia. Photodermatol Photoimmunol Photomed 2006;22:189-92.
[Google Scholar]
|
| 41. |
Pigatto PD, Guzzi G, Schena D, Guarrera M, Foti C, Francalanci S, et al. Photopatch tests: An Italian multicentre study from 2004 to 2006. Contact Dermatitis 2008;59:103-8.
[Google Scholar]
|
| 42. |
Bryden AM, Moseley H, Ibbotson SH, Chowdhury MM, Beck MH, Bourke J, et al. Photopatch testing of 1155 patients: Results of the UK. Multicentre photopatch study group. Br J Dermatol 2006;155:737-47.
[Google Scholar]
|
| 43. |
Pentinga SE, Kuik DJ, Bruynzeel DP, Rustemeyer T. Do 'cinnamon-sensitive' patients react to cinnamate UV filters? Contact Dermatitis 2009;60:210-3.
[Google Scholar]
|
| 44. |
Schauder S, Ippen H. Photoallergic and allergic contact eczema caused by dibenzoylmethane compounds and other sunscreening agents. Hautarzt 1988;39:435-40.
[Google Scholar]
|
| 45. |
Murphy GM, White IR, Cronin E. Immediate and delayed photocontact dermatitis from isopropyl dibenzoylmethane. Contact Dermatitis 1990;22:129-31.
[Google Scholar]
|
| 46. |
Scalf LA, Davis MD, Rohlinger AL, Connolly SM. Photopatch testing of 182 patients: A 6-year experience at the Mayo Clinic. Dermatitis 2009;20:44-52.
[Google Scholar]
|
| 47. |
Lenique P, Machet L, Vaillant L, Bensaid P, Muller C, Khallouf R, et al. Contact and photocontact allergy to oxybenzone. Contact Dermatitis 1992;26:177-81.
[Google Scholar]
|
| 48. |
Szczurko C, Dompmartin A, Michel M, Moreau A, Leroy D. Photocontact allergy to oxybenzone: Ten years of experience. Photodermatol Photoimmunol Photomed 1994;10:144-7.
[Google Scholar]
|
| 49. |
Hughes TM, Stone NM. Benzophenone 4: An emerging allergen in cosmetics and toiletries? Contact Dermatitis 2007;56:153-6.
[Google Scholar]
|
| 50. |
Avenel-Audran M., Dutartre H, Goossens A, Jeanmougin M, Comte C, et al. Octocrylene: An emerging (photo)allergen. Arch Dermatol 2010,;146:753-757.
[Google Scholar]
|
| 51. |
Sommer S, Wilkinson SM, English JS, Ferguson J. Photoallergic contact dermatitis from the sunscreen octyl triazone. Contact Dermatitis 2002;46:304-5.
[Google Scholar]
|
| 52. |
Hughes TM, Martin JA, Lewis VJ, Stone NM. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sun screens. Contact Dermatitis 2005;52:226-7.
[Google Scholar]
|
| 53. |
Gonzalez-Perez R, Trebol I, Garcia-Rio I, Arregui MA, Soloeta R. Allergic contact dermatitis from methylene-bis-benzotriazolyl tetramethylbutylphenol (Tinosorb M). Contact Dermatitis 2007;56:121.
[Google Scholar]
|
| 54. |
Schmidt T, Ring J, Abeck D. Photoallergic contact dermatitis due to combined UVB (4-methylbenzyledine camphor/ octylmethoxy cinnamate) and UVA (benzophenone-3/ butyl methoxydibenzoyl methane) absorber sensitization. Dermatology 1998;196:354-7.
[Google Scholar]
|
Fulltext Views
15,396
PDF downloads
4,449





