Translate this page into:
Correlation of atopic dermatitis with measurement tools in Korean patients: A retrospective study
2 Department of Dermatology, Oregon Health and Science University, Portland, Oregon, USA
3 Department of Dermatology, Chung-Ang University Hospital, Seoul, Korea
Correspondence Address:
Seong Jun Seo
102 Heukseok-Ro, Dongjak-Gu, Seoul 06973
Korea
| How to cite this article: Ahn J, Lee JI, Simpson EL, Seo SJ. Correlation of atopic dermatitis with measurement tools in Korean patients: A retrospective study. Indian J Dermatol Venereol Leprol 2020;86:738-740 |
Sir,
Atopic dermatitis (AD) is a chronic inflammatory skin disease adversely affecting the patient's quality of life (QOL).[1] Current research is focussed on developing diagnostic tools for assessing its severity and possible treatment outcomes. Such tools evaluate their skin lesions, pruritus, sleep dsturbances, depression, anxiety symptoms and QOL,[2],[3]
Some of the tools focus on illness 'as experienced by the patient'.[4] For instance, the Harmonizing Outcome Measures for Eczema (HOME) consensus recommends using Patient-Oriented Eczema Measure (POEM) which reflects the patient's subjective evaluation. According to HOME, POEM should be used as the core outcome tool for evaluating atopic symptoms in all future clinical trials.[4]
The results of a clinical study with dupilumab (Dupixent®) have been published recently; along with emergence of real-world data. This study included a large number of patients from the department of dermatology at National Medical Center (Seoul, the republic of Korea), who were subjected to various evaluation tools simultaneously. The outcome measures of these tools were assessed before and after 16 weeks of dupilumab therapy. This approach helped to identify the precision of assessment tools which reflected the improvement in patient's QOL.
We conducted a retrospective study on AD patients who were administered dupilumab to determine a correlation between the patient assessment tools. The study was approved by our institutional review board. (H-1812-097-001). The patients received treatment with dupilumanb from September 2018 to February 2019 in our hospital. We correlated the Eczema Area Severity Index (EASI), Numeric Rating Scale (NRS), POEM and Dermatologic Life Quality Index (DLQI) at 0 and 16 weeks of dupilumab treatment.
Overall 64 patients were included in ourstudy. Among them males outnumbered females (41:23), their average age being 30.22 years.
The correlations between EASI, NRS, POEM, and DLQI at week 0 were analyzed using pearsons correlation coefficient. The EASI and DLQI scores failed to show any correlation (P = 0.3). POEM and DLQI showed maximum correlation (P < 0.001, r = 0.54), followed by NRS and DLQI (P < 0.001, r = 0.51) [Figure - 1]a. In addition, POEM and NRS (P < 0.001, r = 0.59) and EASI and NRS (P = 0.022, r = 0.3) were correlated, whereas EASI and POEM showed no correlation (P = 0.145, r = 0.19) [Figure - 1]b.
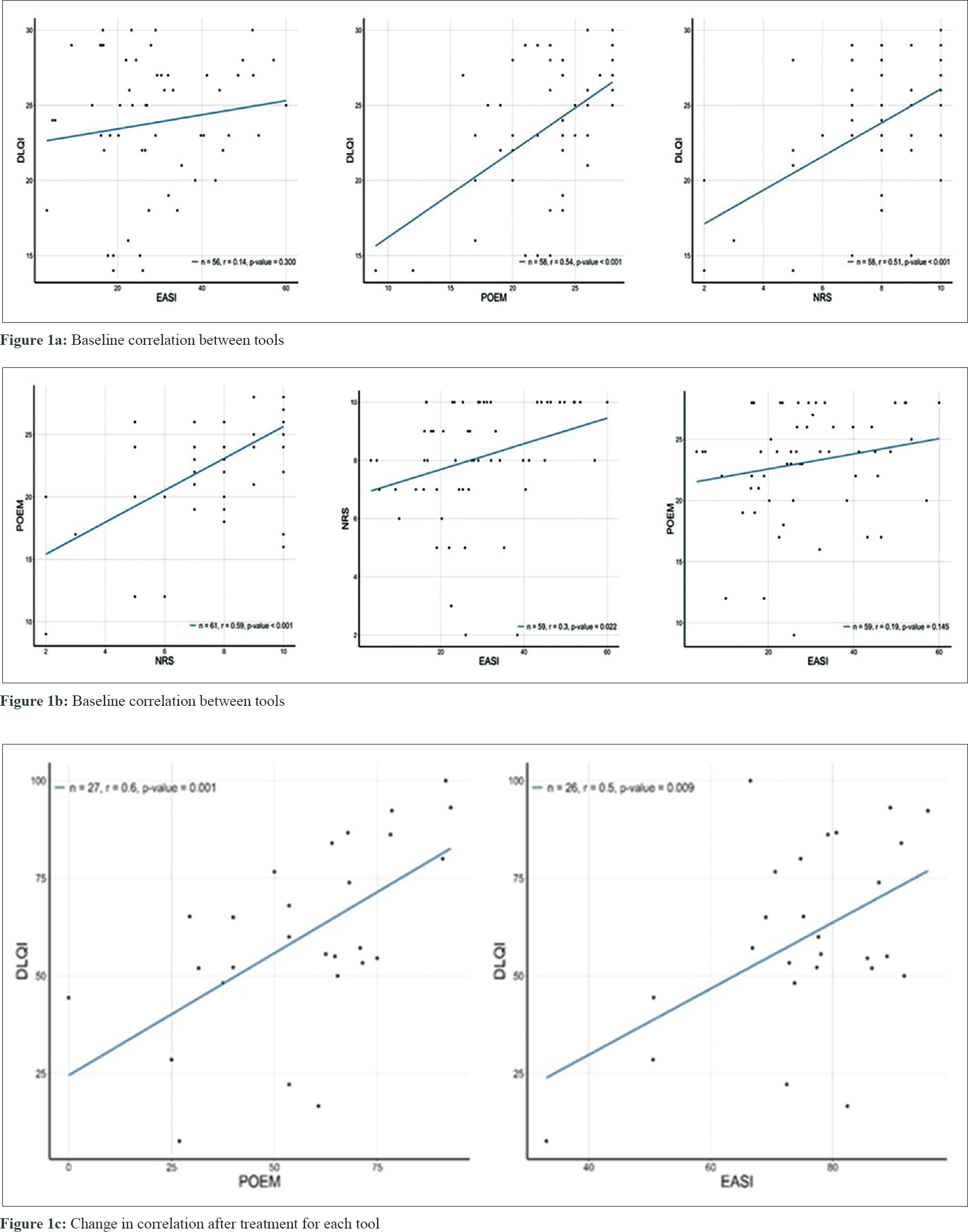 |
Thirty patients (n = 64) completed the 16-week evaluation. We analyzed the changes in correlation for each tool from week 0 to week 16. The changes in POEM assessment showed maximum correlation with changes in DLQI (P = 0.001, r = 0.6), followed by a correlation with changes in EASI (P = 0.009, r = 0.5) [Figure - 1]c.
The EASI scoring system uses a defined process to measure the extent and severity of skin lesions. On the other hand, NRS evaluates patient pruritus, and POEM measures skin lesions, itching, and sleepdisturbances. Therefore, subjective symptoms of AD can be assessed mainly by NRS and POEM.
Similar to AD, newer therapeutic agents are being developed to improve the QOL of patients with psoriasis, and many of these evaluation tools have been used.[5] Subjective symptoms in psoriasis patients are not as common as those with AD. The psoriasis area severity index (PASI) evaluates psoriatic skin lesions and patient's QOL.[5] Takeshita et al. also suggested that the clearance of psoriasis abrogates the negative impact that active psoriasis exerts on a patient's QOL and that a clinically significant improvement in QOL accompanies the transition from 'almost clear' to 'clean' skin.[5]
However, AD patients often report greater satisfaction with treatment than the degree of lesional skin improvement. The association between assessment tools of our patients prior to treatment failed to show any correlation between EASI and quality of life, while POEM and NRS, which included the subjective symptoms, correlated with patients' QQL. In other words, atopic patients experienced a lower quality of life due to pruritus and sleep disturbances, rather than skin lesions alone. The QOL of patients improved maximally when both objective and subjective symptomswere addressed. Our study was limited by a sample size and retrospective study design involving a single centre.
In conclusion, one must not overlook the subjective symptoms of AD while assessing its diagnosis, severity and therapeutic outcomes. POEM for instance, is an useful assessment toolthat evaluates skin lesions and subjective symptoms in a short time. It also reflects the QOL of affected patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014;70:338-51.
[Google Scholar]
|
| 2. |
Schmitt J, Langan S, Williams HC, European Dermato-Epidemiology Network. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 2007;120:1389-98.
[Google Scholar]
|
| 3. |
Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001;10:11-8.
[Google Scholar]
|
| 4. |
Spuls PI, Gerbens LAA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: A Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol 2017;176:979-84.
[Google Scholar]
|
| 5. |
Takeshita J, Callis Duffin K, Shin DB, Krueger GG, Robertson AD, Troxel AB, et al. Patient-reported outcomes for psoriasis patients with clear versus almost clear skin in the clinical setting. J Am Acad Dermatol 2014;71:633-41.
[Google Scholar]
|
Fulltext Views
4,104
PDF downloads
2,132





