Translate this page into:
Cutaneous adverse events of epidermal growth factor receptor inhibitors: A retrospective review of 99 cases
2 Division of Dermatology, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok; Division of Dermatology, Department of Medicine, Thammasart University Hospital, Thammasart University, Pathumthani, Thailand
Correspondence Address:
Vasanop Vachiramon
Division of Dermatology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Rajthevi, Bangkok
Thailand
| How to cite this article: Chanprapaph K, Pongcharoen P, Vachiramon V. Cutaneous adverse events of epidermal growth factor receptor inhibitors: A retrospective review of 99 cases. Indian J Dermatol Venereol Leprol 2015;81:547 |
Abstract
Background: Previous reports regarding the cutaneous adverse events of epidermal growth factor receptor inhibitors are mostly limited to small case reports and case series, mainly involving Caucasian patients. Aims: We describe the trends in the clinical presentation of Asian patients who had cutaneous adverse events induced by epidermal growth factor receptor inhibitors and to explore the relationship between skin adverse events and tumor response. Methods: From 2006 to 2010, medical records of Thai patients with non-small cell lung cancer receiving epidermal growth factor receptor inhibitors were retrieved and analyzed. Results: In all, 99 patients were reviewed and analyzed. Erlotinib and gefitinib were commenced in 75 (75.8%) and 24 (24.2%) patients, respectively. Cutaneous adverse events occurred in 43 (57.3%) patients receiving erlotinib and in 15 (62.5%) patients receiving gefitinib. The most common adverse event was xerosis (52.5%). Less common adverse events included papulo-pustular eruption (27.3%), erythematous maculopapular rash (11.1%), mucositis (6.7%), paronychia (5.1%), and trichomegaly (2%). Elderly patients had a higher occurrence of xerosis. The presence of cutaneous adverse events was significantly higher in subjects who had a tumor response. Limitations: The limitations of study include its retrospective nature, and the initial screening of cutaneous adverse events was done by non-dermatologists. Conclusions: Cutaneous adverse events due to epidermal growth factor receptor inhibitors are not uncommon in the Asian population. We found a positive correlation between the occurrences of cutaneou adverse events and tumor response supporting the view that they are surrogate markers for therapeutic response.INTRODUCTION
The epidermal growth factor receptor has become an important target for cancer therapy. These agents are currently in widespread use for advanced malignancy as they have improved ability to target cancers cells and an enhanced safety profile compared to conventional chemotherapies. [1],[2] Despite the benefits, targeted chemotherapies have enormous skin adverse events which may lead to poor adherence, dose interruption, and discontinuation of these therapeutic regimens. Moreover, psycho-social discomfort leading to reduction in the quality of life can frequently occur. Patients undergoing treatment with epidermal growth factor receptor inhibitors frequently present with a distinct cutaneous adverse event that results from interference of epidermal growth factor receptor signaling in the skin. Previous reports of the incidence of cutaneous adverse events from EGFR inhibitors indicate these to be present in 80% of patients. [3] The presence and severity of rash are indicators of tumor response as well as overall survival. Nevertheless, most studies have been done in non-Asian patients. [4] The aim of this study is to evaluate clinical features of cutaneous adverse events of epidermal growth factor receptor inhibitors and to explore the relationship between skin adverse events and tumor response in Asians.
METHODS
The study was conducted with approval from the Mahidol University Institutional Review Board for Human Subject Research (Protocol number 09-52-38). Patients with advanced non-small cell lung cancer from the oncology unit of a tertiary care centre in Thailand (Ramathibodi Hospital, Mahidol University, Bangkok) who received erlotinib or gefitinib monotherapy and developed cutaneous adverse events were referred to the dermatology clinic. Three dermatologists evaluated the patients and recorded their dermatologic findings in the medical record. The medical records of patients treated from January 2006 to December 2010 were retrospectively reviewed. Demographic data, dermatologic adverse events, interval between drug initiation and the development of adverse events, and tumor response were collected and analyzed. The severity of skin adverse events was graded according to the National Cancer Institute′s Common Terminology Criteria for Adverse Event v4.0 (NCI-CTCAE v4.0). [5] Tumor response to epidermal growth factor receptor inhibitors was evaluated by following the Response Evaluation Criteria In Solid Tumors (RECIST). [6] Patients′ response to therapy was defined as a 30% tumor size reduction. Tumor progression was defined by 20% increase in sums of the longest tumor diameters.
The statistical analyses were performed using computer software. All data were entered and analyzed in SPSS (version 17.0; SPSS Inc, Chicago, IL, USA). Categorical variables (i.e., gender, type of cutaneous toxicity) were expressed as percentages, while continuous variables (i.e., age, latent periods) were expressed in terms of mean ± standard deviation. Correlation between cutaneous adverse events and tumor response was determined using the Chi-square test. P < 0.05 was considered statistically significant.
RESULTS
Demographic characteristics
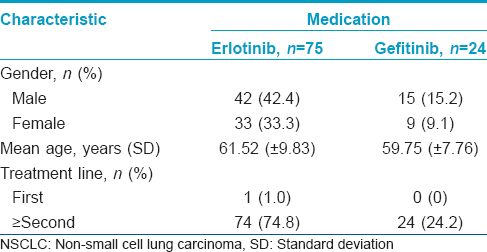
In all, 99 patients (57 males and 42 females) received epidermal growth factor receptor inhibitors in this 5-year retrospective study. All subjects had advanced non-small cell lung cancer with failure to respond to standard chemotherapeutic regimens. Seventy-five (75.8%) patients received erlotinib 150 mg/day orally and 24 (24.2%) patients received gefitinib 250 mg/day orally. In the erlotinib group, the mean age was 61.52 (±9.83). The mean age of patients in the gefitinib group was 59.75 (±7.76). The mean duration of treatment was 9.3 months for erlotinib and 8.4 months for gefitinib. The mean observation period was 5.2 months after the first visit to the dermatology clinic. There was no statistically significant difference in all demographic data between the two groups [Table - 1].
Cutaneous adverse events
Cutaneous adverse events were found in 68 (68.7%) patients. Among 75 patients receiving erlotinib, 53 (70.7%) patients developed skin adverse events. In gefitinib group, 15 out of 24 patients (62.5%) developed cutaneous adverse event. The most common adverse event in both the groups was xerosis [Figure - 1], with 42 patients (56.0%) in the erlotinib group and 10 patients (41.7%) in the gefitinib group. The second most common adverse event was papulo-pustular eruption [Figure - 2], with 22 (29.3%) patients in the erlotinib group and 5 (20.8%) in gefitinib group. Less common adverse events were maculopapular rash, mucositis, paronychia, and trichomegaly of the eyelashes [Table - 2], [Figure - 3] and [Figure - 4]. The prevalence of each type of cutaneous adverse event between the erlotinib and gefitinib group was not different statistically (data not shown).
 |
| Figure 1: Xerosis. Ill-defined dry scaly patch with mild erythema on the right upper arm which occurred 3 weeks following gefitinib |
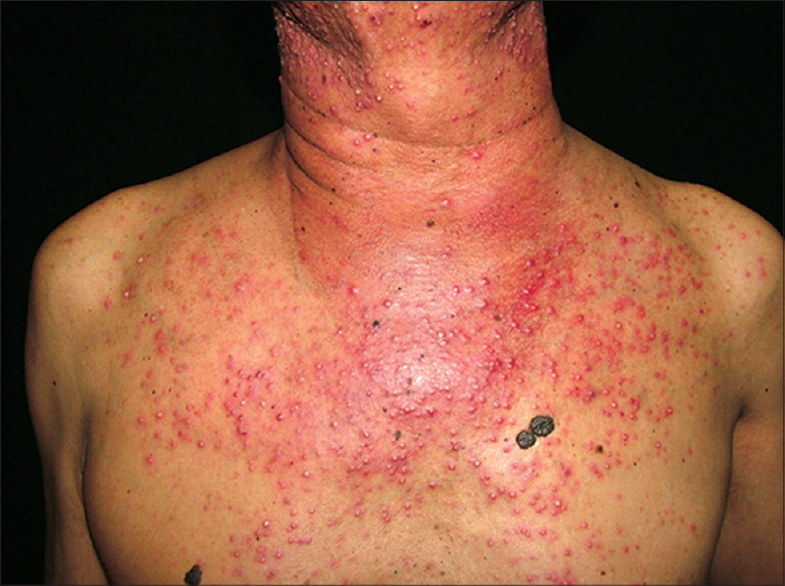 |
| Figure 2: Papulo-pustules. A 52-year-old male with underlying non-small cell lung carcinoma stage IV developed papulo-pustules after 10 days erlotinib therapy |
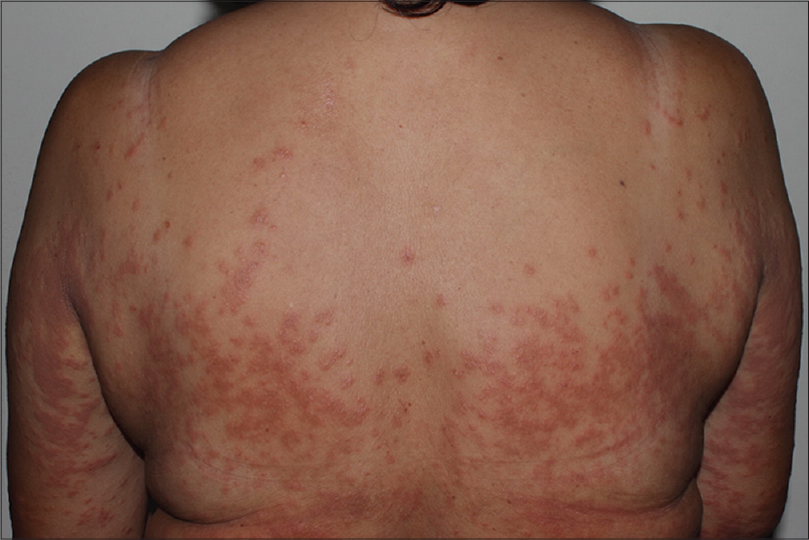 |
| Figure 3: Maculopapular rash. A 45-year-old female presented with erythematous maculopapular rash 2 weeks after gefitinib was commenced |
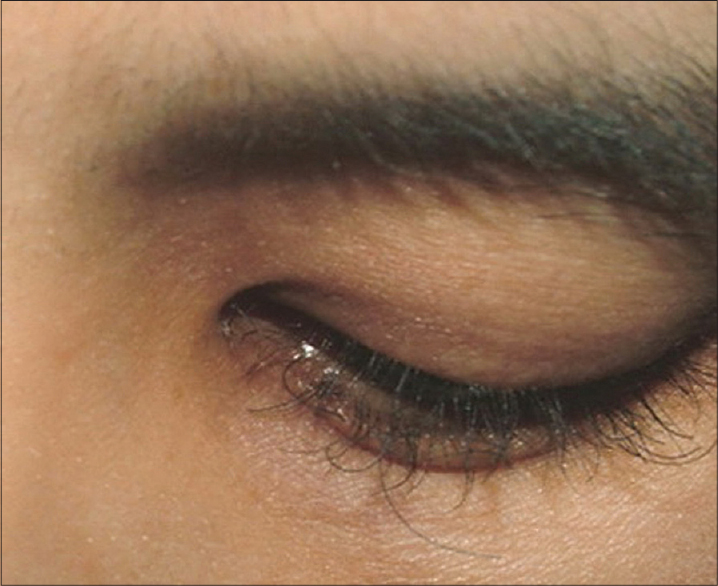 |
| Figure 4: Trichomegaly. Trichomegaly appeared 3 months after erlotinib was administered. Notice the uneven, curly, and aberrant elongation of the eyelashes |
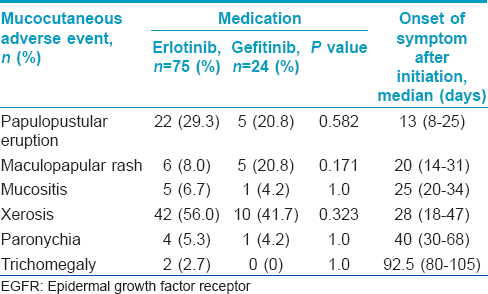
Regarding the relationship between demographic characteristics and the occurrence of cutaneous adverse events, the mean age of patients who developed xerosis was significantly higher than those who did not develop xerosis (mean age 63.88 ± 8.34 vs. 58.00 ± 9.55, P = 0.001). There were no significant differences between mean age and the presence of other cutaneous adverse events [Table - 3]. Gender had no impact on the occurrence of cutaneous adverse events.

Severity of cutaneous adverse events
Among 27 patients who developed a papulo-pustular eruption, 15 (55.6%) patients had grade 1 severity (less than 10% body surface area) according to the NCI-CTCAE v4.0. Grade 2 severity (10-30% body surface area) was found in 10 patients, whereas grade 3 severity (>30% body surface area) was found in 2 patients.
Latent period
The time period from starting epidermal growth factor receptor inhibitors to the onset of cutaneous adverse events ranged from 8 days to 15 weeks, depending on the type of cutaneous adverse event. The earliest cutaneous adverse event was papulo-pustular eruption for which the median latent period was 13 days (8-25). Maculopapular rash was observed after a median latent period of 20 days (14-31). Mucositis and xerosis developed at 25 (20-34) and 28 (18-47) days after the treatment, respectively. Paronychia appeared 30-68 days (median 40 days) after initiation of the treatment. Trichomegaly was observed at 3 months of therapy [Table - 2].
Tumor response
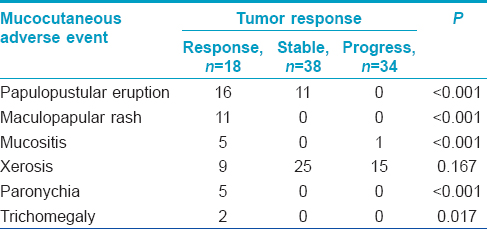
Eighteen (20.0%) out of 90 patients had tumor response, 38 (42.2%) had stable disease and 34 (37.8%) had tumor progression. Nine remaining patients were not included in the analysis due to the lack of follow-up data. We found a significantly greater tumor response in patients who had two or more different types of cutaneous adverse events compared to those having one or none (P < 0.001) [Table - 3]. Regarding the type of cutaneous adverse event and tumor response, papulo-pustular eruption, maculopapular rash, mucositis, paronychia, and trichomegaly were associated with tumor response while skin dryness was not [Table - 4].
Treatment
Patients with xerosis were treated with emollients (e.g., urea preparations and petroleum jelly) with improvement in all patients. Grade 1 papulo-pustular eruption was managed by medium-potency topical corticosteroids and benzoyl peroxide. Patients with grade 2 and 3 severity were treated with topical corticosteroids, benzoyl peroxide and oral doxycycline (200 mg/day). Trichomegaly was treated by eyelash trimming. Skin side effects led to transient dose interruption in 10 (10.1%) patients, and 3 (3%) patients underwent dose reductions. Severe papulo-pustular eruption was the major cause of treatment interruption.
DISCUSSION
Epidermal growth factor receptor inhibitor are currently in widespread use for advanced malignancies. Patients undergoing epidermal growth factor receptor inhibitor therapy frequently presented with cutaneous adverse events that result from interference of epidermal growth factor receptor signaling. Epidermal growth factor receptor is highly expressed in undifferentiated and proliferating basal keratinocytes, sebocytes and outer root sheath of hair follicle, which rely on epidermal growth factor receptor to regulate proliferation, differentiation, migration and survival. [7] Therefore, clinically distinct patterns of cutaneous adverse events can be observed from alteration of the normal function of these structures. Cutaneous adverse events have been observed with all agents that target epidermal growth factor receptor and is therefore considered a class specific effect. In addition, the frequency and severity of skin rashes are dose dependent. [8],[9],[10],[11] Therefore, gradual dose increment until the skin eruption appears is a strategy to maximize efficacy of epidermal growth factor receptor inhibitors. The wide spectrum of cutaneous adverse events has been in termed the PRIDE syndrome (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to epidermal growth factor receptor inhibitors). [12] These effects are generally mild to moderate in severity but may negatively affect the quality of life and lead to treatment delays, dose modifications and discontinuation of therapy. [13],[14] Numerous retrospective studies have found that the presence and severity of rash are indicators of tumor response as well as overall survival. [4]
This study describes the clinical characteristics of cutaneous adverse events in 99 Asian patients treated with erlotinib and gefitinib. Sixty-eight percent of patients in the present study experienced at least one cutaneous symptom. In our study, xerosis was the most common adverse event, occurring up to 52.5%. Because epidermal growth factor is required to maintain the normal epithelial barrier function, its inhibition results in dryness. Xerosis was particularly more prevalent in the older age group. In contrast, in a recent publication by Nakagawa et al., [15] the incidence of xerosis among Asian patients treated with erlotinib was 7.7%. The vast difference may be from the fact that all our patients had prior therapy with various cytotoxic drugs leading to skin barrier alteration. It is also a common practice among Thai patients to use soap bars as body cleansers. Moreover, due to the hot climate, Thai people often take showers many times per day. All these factors promote skin dryness. According to a study by Osio et al., [16] the incidence of xerosis among Caucasians was up to 100% after 6-month treatment. The variation in the reported incidences of xerosis compared with our study may possibly be from different durations which epidermal growth factor receptor inhibitors was administered. Moreover, there are no standard criteria to define xerosis. This could also be the reason for the vast difference in the reported incidence in the literature.
Unlike previous reports where the incidence of papulo-pustular eruption was up to 86%, [15],[16],[17],[18] our study showed that papulopustular eruption occurred in 27.3% patients. This could be explained by variable classifications as well as indistinct descriptions this cutaneous adverse event. Many studies refer to epidermal growth factor receptor inhibitor-associated folliculitis as "rash", "acne", "acneiform eruption", "papulo-pustular eruption", "folliculitis", and "maculopapular skin rash". The classic presentation of papulo-pustular eruption is inflamed papules or pustules with hair at the center. It can be preceded by pruritus and dysesthesia for a few days. In our study, papulo-pustular eruption was clearly differentiated from maculopapular rash. Maculopapular rash would appear as macular erythema and slightly elevated erythematous coalescing papules lacking folliculocentric pustules. In addition, the maculopapular rashes in our patients resolved spontaneously in 1-2 weeks. This differs from papulo-pustular eruption, which developed in 1-2 weeks, peaked at 3-4 weeks and decreased in intensity over several weeks leaving mild erythema and follicular papules throughout the course of therapy. [19] The inflamed and itchy nature also helps differentiate epidermal growth factor receptor inhibitor-induced papulo-pustules from similar eruption caused by other drugs. The typical drug-induced acneiform eruption from agents such as corticosteroids, antiepileptic, vitamin B complexes, and antituberculosis drug is usually non-pruritic and non-inflammatory. [20],[21] The time of occurrence and the time to resolution of the papulo-pustular eruption were similar to Caucasian patients. [3] In our study, we found that papulo-pustular eruption was the earliest to occur, with a median onset of 13 days.
Patients in the erlotinib group had a tendency toward greater cutaneous adverse events compared with the gefitinib group. This could be due to differences in the molecular structures of these two agents, leading to variation in pharmacokinetic properties. Gefitinib 250 mg/day has a lower mean plasma concentration and area under the plasma drug concentration-time curve compared with erlotinib 150 mg/day. Moreover, gefitinib has higher volume distribution and clearance leading to a greater rate of elimination. [22],[23] Drug tumor tissue accumulation is also higher in gefitinib. [22],[24] In addition, gefitinib provides the concentration needed to achieve effective epidermal growth factor receptor inhibition in the tumor at a dose below the maximum tolerated dose. [22] Thus, erlotinib is administered at the maximal tolerated dose, whereas gefitinib is efficient at one-third of the maximal tolerated dose. Therefore, the frequency of skin adverse events, which increases with the dose of epidermal growth factor receptor inhibitors, is greater with erlotinib than with gefitinib. [24]
Trichomegaly of the eyelashes was observed in 2% in our study. This is an uncommon adverse event but can represent an important cause of esthetic damage. Moreover, it can be complicated by trichiasis and secondary corneal ulceration. Our patients required periodic trimming of the eyelashes while the drugs were administered. This adverse event could last for a long period after treatment discontinuation.
A positive correlation between the occurrence of cutaneous adverse events and tumor response was observed. This finding was similar to previous studies in Caucasians. [4],[25],[26] This correlation has also been observed in patients with squamous cell carcinoma of the head and neck or metastasized colorectal cancer treated with epidermal growth factor receptor inhibitors. [27],[28],[29] We also found that individuals experiencing multiple cutaneous adverse events had a better therapeutic outcome compared to those with a single skin adverse event.
We used topical triamcinolone and topical benzoyl peroxide to treat the papulo-pustular eruption. Oral doxycycline was administered with reasonable improvement in patients experiencing grade 2 and 3 rash severity. Recently, Garber et al. [30] reported that not only were anti-inflammatory and anti-infectious agents effective but a combined triple therapy comprising of topical prednicarbate cream, topical nadifloxacin cream and systemic isotretinoin also offered significant improvement of the rash severity score. Systemic isotretinoin was not administered in our study because it could worsen xerosis.
The limitations of this study include its retrospective nature, leading to recall bias and incomplete reporting of data. In addition, all information was collected from patients who were referred by oncologists for dermatologic consultation. Therefore, some of the clinical manifestations may have been missed, leading to an impression of a lower incidence of cutanous adverse events. Future prospective studies are essential to validate these results.
In conclusion, cutaneous toxicities from epidermal growth factor receptor inhibitors are common among the Asian population. Xerosis was the most prevalent cutaneous adverse event in this study. We found that with numerous cutaneous adverse events, patients had better therapeutic outcome. It is important for practitioners to know about the cutaneous adverse event of epidermal growth factor receptor inhibitors in order to provide optimum management and allow patients to remain on these life prolonging therapies.
| 1. |
Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160-74.
[Google Scholar]
|
| 2. |
Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res 2006;12:7232-41.
[Google Scholar]
|
| 3. |
Peuvrel L, Bachmeyer C, Reguiai Z, Bachet JB, André T, Bensadoun RJ, et al. Semiology of skin toxicity associated with epidermal growth factor receptor (EGFR) inhibitors. Support Care Cancer 2012;20:909-21.
[Google Scholar]
|
| 4. |
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan DM, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: A systematic review and meta-analysis. PLoS One 2013;8:e55128.
[Google Scholar]
|
| 5. |
Common Terminology Criteria for Adverse Event (CTCAE) v4.0. US Department of Health and Human Services. National Institute of Health. National Cancer Institute. May 28, 2009. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_Quickreference_5×7.pdf. [Last accessed on 2013 Apr 17].
[Google Scholar]
|
| 6. |
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16.
[Google Scholar]
|
| 7. |
Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 2005;16:1425-33.
[Google Scholar]
|
| 8. |
Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006;55:657-70.
[Google Scholar]
|
| 9. |
Jacot W, Bessis D, Jorda E, Ychou M, Fabbro M, Pujol JL, et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol 2004;151:238-41.
[Google Scholar]
|
| 10. |
Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol 2009;4:107-19.
[Google Scholar]
|
| 11. |
Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: A review of cutaneous adverse events and management. Dermatol Res Pract 2014;2014:734249.
[Google Scholar]
|
| 12. |
Lacouture ME, Lai SE. The PRIDE (Papulopustules and/or paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to Epidermal growth factor receptor inhibitors) syndrome. Br J Dermatol 2006;155:852-54.
[Google Scholar]
|
| 13. |
Wagner LI, Lacouture ME. Dermatologic toxicities associated with EGFR inhibitors: The clinical psychologist′s perspective. Impact on health-related quality of life and implications for clinical management of psychological sequelae. Oncology (Williston Park) 2007;21:34-6.
[Google Scholar]
|
| 14. |
Boone SL, Rademaker A, Liu D, Pfeiffer C, Mauro DJ, Lacouture ME, et al. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: Survey results. Oncology 2007;72:152-9.
[Google Scholar]
|
| 15. |
Nakagawa K, Kudoh S, Ohe Y, Johkoh T, Ando M, Yamazaki N, et al. Postmarketing surveillance study of erlotinib in Japanese patients with non-small-cell lung cancer (NSCLC): An interim analysis of 3488 patients (POLARSTAR). J Thorac Oncol 2012;7:1296-303.
[Google Scholar]
|
| 16. |
Osio A, Mateus C, Soria JC, Massard C, Malka D, Boige V, et al. Cutaneous side-effects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br J Dermatol 2009;161:515-21.
[Google Scholar]
|
| 17. |
Balagula Y, Garbe C, Myskowski PL, Hauschild A, Rapoport BL, Boers-Doets CB, et al. Clinical presentation and management of dermatological toxicities of epidermal growth factor receptor inhibitors. Int J Dermatol 2011;50:129-46.
[Google Scholar]
|
| 18. |
Shin JU, Park JH, Cho BC, Lee JH. Treatment of epidermal growth factor receptor inhibitor-induced acneiform eruption with topical recombinant human epidermal growth factor. Dermatology 2012;225:135-40.
[Google Scholar]
|
| 19. |
Abdullah SE, Haigentz M Jr., Piperdi B. Dermatologic toxicities from monoclonal antibodies and tyrosine kinase inhibitors against EGFR: Pathophysiology and management. Chemother Res Pract 2012;2012:351210.
[Google Scholar]
|
| 20. |
Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: Clinical presentation, pathogenesis, and management. J Am Acad Dermatol 2007;56:317-26.
[Google Scholar]
|
| 21. |
Madke B, Gole P, Kumar P, Khopkar U. Dermatological Side Effects of Epidermal Growth Factor Receptor Inhibitor: PRIDE Complex. Indian J Dermatol 2014;59:271-4.
[Google Scholar]
|
| 22. |
Rukazenkov Y, Speake G, Marshall G, Anderton J, Davies BR, Wilkinson RW, et al. Epidermal growth factor receptor tyrosine kinase inhibitors: Similar but different? Anticancer Drugs 2009;20:856-66.
[Google Scholar]
|
| 23. |
van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 2009;35:692-706.
[Google Scholar]
|
| 24. |
Wolf M, Swaisland H, Averbuch S. Development of the novel biologically targeted anticancer agent gefitinib: Determining the optimum dose for clinical efficacy. Clin Cancer Res 2004;10:4607-13.
[Google Scholar]
|
| 25. |
Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol 2004;22:3238-47.
[Google Scholar]
|
| 26. |
Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 2007;13:3913-21.
[Google Scholar]
|
| 27. |
Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol 2005;23:8646-54.
[Google Scholar]
|
| 28. |
Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004;22:77-85.
[Google Scholar]
|
| 29. |
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17.
[Google Scholar]
|
| 30. |
Gerber PA, Meller S, Eames T, Buhren BA, Schrumpf H, Hetzer S, et al. Management of EGFR-inhibitor associated rash: A retrospective study in 49 patients. Eur J Med Res 2012;17:4.
[Google Scholar]
|
Fulltext Views
5,160
PDF downloads
3,129





