Translate this page into:
Cutaneous lymphoid hyperplasia following vaccine for pollen hyposensitization
2 Department of Pathology, San Cecilio University Hospital, Granada, Spain
Correspondence Address:
Mar�a Librada Porri�o-Bustamante
Department of Dermatology, San Cecilio University Hospital, Av. Doctor Olóriz, 16, 18012 Granada
Spain
| How to cite this article: Porri�o-Bustamante ML, Aneiros-Fern�ndez J, Ret�mero JA, S�nchez-L�pez J, Fern�ndez-Pugnaire MA. Cutaneous lymphoid hyperplasia following vaccine for pollen hyposensitization. Indian J Dermatol Venereol Leprol 2016;82:193-195 |
Sir,
Cutaneous lymphoid hyperplasia is also known as cutaneous lymphocytoma. Cutaneous lymphoid hyperplasia belongs to the spectrum of cutaneous pseudolymphomas which are lymphocytic infiltrates with clinical or histological features that mimic cutaneous lymphomas but follow a benign course. Most of these are predominantly composed of B cells. [1]
Cutaneous lymphoid hyperplasia is usually idiopathic but sometimes is associated with arthropod bites, scabies, infections (Borrelia burgdorferi is the most commonly identified agent in Europe), drugs, tattoo pigments and vaccine residues. [1],[2] It is more frequent in middle aged Caucasian women (sex ratio F:M 3:1). [1]
Adverse effects of vaccination are usually benign and transient, generally consisting of mild erythema or pain at the site of injection. Less commonly, chronic papules or subcutaneous nodules may appear which have been related to aluminum-adsorbed vaccines. [1],[2] Some rare cases of cutaneous lymphoid hyperplasia secondary to hyposensitization vaccines have been reported. [3]
A 26-year-old Caucasian woman presented with itchy nodules located at the lateral side of both arms. The itchy nodules had appeared 12 years back but had recently become increasingly itchy. She was allergic to olive tree pollen and the nodules had developed at the site of hyposensitization vaccine injections against olive tree pollen that she had received during childhood. The vaccination course consisted of an induction period during which she received a weekly subcutaneous injection for 3 months followed by monthly injections for 3 years. These nodules appeared 3 years after commencing the vaccination course; for this reason, the treatment was discontinued.
Physical examination revealed five subcutaneous nodules that were mobile, firm and showed excoriation marks [Figure - 1]a. The nodules were painless and the overlying skin had an erythemato-violaceous hue. The complete blood count and biochemistry tests were normal. Serology for Borrelia burgdorferi was negative. A punch biopsy revealed a nodular lymphoid infiltrate with enlarged germinal centers located in the deep dermis and hypodermis [Figure - 1]b where apoptotic bodies were also observed [Figure - 1]c. Giant multinucleated cells were also seen. [Figure - 2]a. In addition, an inflammatory interstitial infiltrate with several eosinophils was noted in the upper dermis [Figure - 2]b. Immunohistochemistry showed a predominantly B-lymphocytic infiltrate (CD20+). Germinal centers of lymphoid follicles were positive for CD21 and CD23 and negative for Bcl-2 [Figure - 3]a but the periphery of the follicles was positive for Bcl-2. The germinal centers also showed positivity for Bcl-6 [Figure - 3]b. Follicles were negative for cyclin D1. There was no rearrangement for B lymphocytic markers (IgH, IgK-IgL).
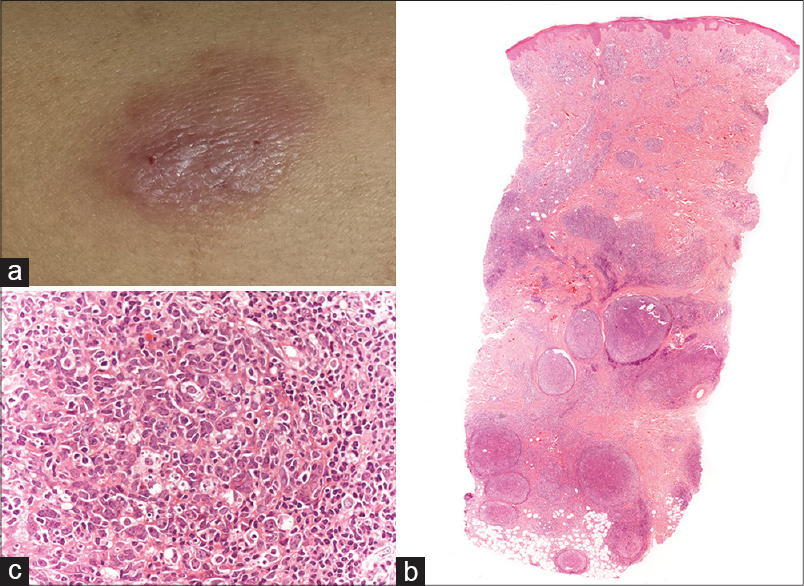 |
| Figure 1: (a) One of several subcutaneous mobile and firm nodules located on the arms and accompanied by excoriations.(b) Dense nodular infiltrate of lymphocytes with follicular pattern (H and E, ×10). (c) Apoptotic bodies in the germinal centre (H and E, ×400) |
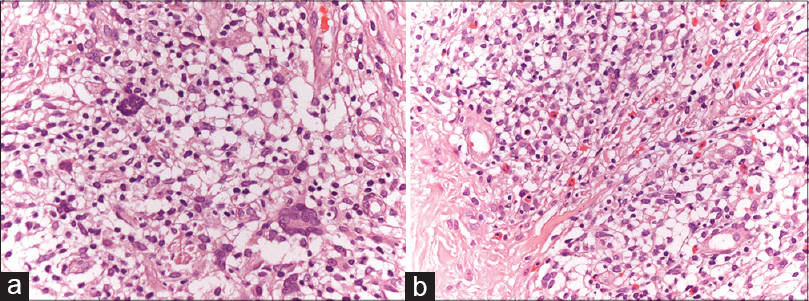 |
| Figure 2: (a) Giant multinucleated cells (H and E, ×400). (b) Inflammatory interstitial infiltrate with several eosinophils in the upper dermis (H and E, ×400) |
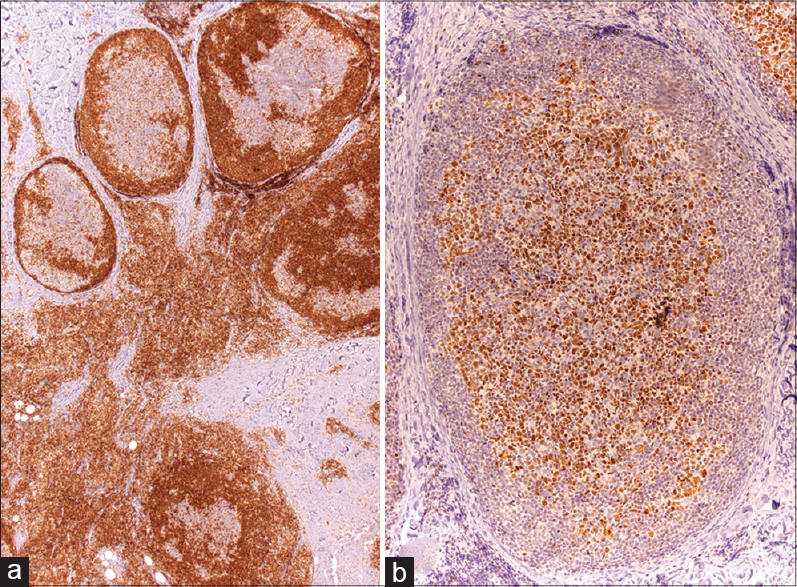 |
| Figure 3: (a) Staining with Bcl-2 showed negativity for germinal centers (×400). (b) Germinal center cells were positive for Bcl-6 (×200) |
The diagnosis of cutaneous lymphoid hyperplasia due to hyposensitization vaccine was made considering the evolution and distribution of the lesions and in the absence of other etiological factors. Treatment with intralesional and topical corticosteroids resulted only in a slight improvement in pruritus.
Cutaneous lymphoid hyperplasia related to aluminum-adsorbed vaccines is a rare occurrence. It has been described in association with vaccines for influenza, hepatitis A and B, meningoencephalitis, diphtheria and tetanus. [1],[2],[4] A few cases due to hyposensitization vaccine have been reported. [3] Our patient had received vaccines that contained aluminum hydroxide as an adjuvant.
Clinically, patients present with infiltrative lesions at the site of injection, usually in the arm or shoulder that appear weeks or even years after vaccination and with variable duration. [1],[2]
Biopsy shows a histiocytic foreign body reaction with subsequent giant cells and central necrosis. A B-cell lymphoid infiltrate arranged in follicles with large germinal centers can be seen located mainly in the subcutaneous fat. [2] The lymphoid infiltrate may reach deeper in vaccination-induced cutaneous lymphoid hyperplasia than in cutaneous lymphoid hyperplasia due to other causes, maybe because of the injection technique. [1] In these cases, aluminum deposits are frequently found.
Differential diagnoses include B-cell follicular lymphoma, Jessner lymphocytic infiltrate and lupus panniculitis. The differentiation between cutaneous lymphoid hyperplasia and cutaneous lymphoma is often difficult. The morphologic change that favors a diagnosis of follicular hyperplasia versus follicular lymphoma is mainly the presence of hypertrophic germinal centers with well-defined mantle zones. These centers should show a variable proportion of centroblasts and centrocytes with abundant histiocytic cells phagocytosing apoptotic bodies. Our case, in addition, showed giant multinucleated cells and eosinophils that would also indicate a reactive process. Other findings that support the diagnosis of follicular hyperplasia are the negativity for Bcl-2 and the lack of B rearrangement (IgH). However, malignant transformation has been reported. Therefore, close long-term follow-up is needed. [4]
Several treatments have been proposed for persistent lesions including oral doxycycline, corticosteroids, radiotherapy, cyclosporine, surgical removal, phototherapy and intralesional rituximab. [5] In our patient, corticosteroids produced a slight symptomatic relief and surgery was not suitable due to the extent of the lesions. Treatment with intralesional rituximab was contraindicated as the patient planned to get pregnant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Pham-Ledard A, Vergier B, Doutre MS, Beylot-Barry M. Disseminated cutaneous lymphoid hyperplasia of 12 years′ duration triggered by vaccination. Dermatology 2010;220:176-9.
[Google Scholar]
|
| 2. |
Cerroni L, Borroni RG, Massone C, Chott A, Kerl H. Cutaneous B-cell pseudolymphoma at the site of vaccination. Am J Dermatopathol 2007;29:538-42.
[Google Scholar]
|
| 3. |
Chong H, Brady K, Metze D, Calonje E. Persistent nodules at injection sites aluminium granuloma clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology 2006;48:182-8.
[Google Scholar]
|
| 4. |
Atalar H, Sarifakioglu E, Dener C, Yanik B, Koktener A, Bayrak R. Cutaneous lymphoid hyperplasia and reactive lymphadenopathy induced by hepatitis B vaccination. Eur J Dermatol 2008;18:188-9.
[Google Scholar]
|
| 5. |
Martin SJ, Duvic M. Treatment of cutaneous lymphoid hyperplasia with the monoclonal anti-CD20 antibody rituximab. Clin Lymphoma Myeloma Leuk 2011;11:286-8.
[Google Scholar]
|
Fulltext Views
3,777
PDF downloads
3,141





