Translate this page into:
Cutaneous mucormycosis of scalp and eyelids in a child with type I diabetes mellitus
2 Department of Pediatric Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India
3 Department of Plastic Surgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Shivaprakash M Rudramurthy
Additional Professor, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012
India
| How to cite this article: Zaman K, Kaur H, Rudramurthy SM, Singh M, Parashar A, Chakrabarti A. Cutaneous mucormycosis of scalp and eyelids in a child with type I diabetes mellitus. Indian J Dermatol Venereol Leprol 2015;81:275-278 |
Abstract
Scalp mucormycosis in children is extremely rare. We present a case of pediatric scalp mucormycosis caused by Rhizopus oryzae in a 9-year-old diabetic girl who was successfully diagnosed and treated with amphotericin B deoxycholate and wound debridement. At 3 months follow up, the patient was stable although she had lost her vision.INTRODUCTION
Mucormycosis is a fungal infection with a high mortality rate in unrecognized or untreated cases. [1] Primary cutaneous mucormycosis (PCM) develops following a breach in the skin and is seen mainly in adults, with occasional cases in children. [1] Primary cutaneous mucormycosis commonly affects the extremities in an immunocompromised individual and is usually acquired nosocomially. [2] However, cutaneous mucormycosis involving other regions like the face, nose, breast, chest, back, abdomen, gluteal area, perineum, and scalp have been reported earlier. [3],[4],[5],[6] We report a case of pediatric primary cutaneous mucormycosis affecting the scalp, forehead, and upper eyelids, which was promptly diagnosed and successfully treated.
Case Report
A 9-year-old girl with a four-year history of type I diabetes mellitus, on regular insulin, developed a painful, oozing lesion of 3 × 3 cm size on her scalp, following a minor fall, which enlarged over time and turned gangrenous within 6 days. A local practitioner debrided the wound and instituted antibiotics (piperacillin-tazobactam). She did not respond to the treatment and within two weeks, the gangrene extended across her entire forehead, temporal area and both upper eyelids, at which point, she visited the emergency department of Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. At presentation, the fronto-temporal region, roof of the nose and both upper eyelids appeared gangrenous (dry type), exposing the underlying bones and impairing eye closure. Vision was blurred due to exposure keratitis. Ophthalmic examination revealed gangrene of the entire right upper eyelid and two-thirds of the left upper eyelid accompanied by pus discharge and corneal opacities, bilaterally [Figure - 1]. Laboratory investigations at admission revealed a raised total leukocyte count (15,900 cells/μL), elevated random blood glucose level (300 mg/dl) and the presence of ketone bodies in the urine. Computed tomography (CT) of the skull showed thickening of the fronto-temporal scalp. CT of the paranasal sinuses (PNS) did not show any involvement of sinuses, brain, or optic nerve. Cutaneous mucormycosis, necrotizing fasciitis and ecthyma gangrenosum were the differential diagnoses considered. Necrotic tissue from the scalp and eyelids was sent for mycological investigation. Potassium hydroxide (KOH) mount showed broad aseptate hyphae with right angle branching [Figure - 2]. The tissue was inoculated on Sabouraud dextrose agar (SDA) containing chloramphenicol and gentamicin and was incubated at 25°C and 37°C. On the third day of incubation, cotton candy-like mouldy growth with black spores and salt-and-pepper appearance was observed [Figure - 3]a. Lactophenol cotton blue (LCB) mount from the growth revealed aseptate hyphae with nodal rhizoids, and unbranched sporangiophores with terminal spherical sporangia filled with brownish rhomboidal sporangiospores, suggesting it to be Rhizopus oryzae [Figure - 3]b. Molecular diagnosis was carried out directly from the tissue specimen by polymerase chain reaction (PCR) and sequencing using panfungal primers ITS1 (5′-TCCGTAGGTGAA CCT GCGG-3′) and ITS4 (5′- TCC TCC GCT TAT TGA TATGC-3′). [7],[8],[9] NCBI BLAST search of our sequence showed 99% homology with standard strain of Rhizopus oryzae CBS 395.95 (Genbank accession number AY213684). The isolate was deposited in the National Culture Collection of Pathogenic Fungi (NCCPF), PGIMER, Chandigarh, India with the accession number NCCPF 710201. The nucleotide sequence was deposited to GenBank with the accession number KM225290. The scalp and eyelid gangrene were surgically debrided along with bilateral tarsorraphy to prevent progression of corneal opacities. The local wound was treated by regular dressing. Intravenous amphotericin B deoxycholate was started at a dose of 1 mg/kg, which was later increased to 1.5 mg/kg for 14 days. Regular insulin was started along with a diabetic diet. Reconstructive surgery of the affected area was done during follow-up with a split skin graft from the right thigh, which was taken up well. However, extensive corneal opacities rendered the child blind [Figure - 4]. She was discharged and advised to follow-up for further reconstructive surgery. At 3-months follow-up, she is well with no recurrence of the disease.
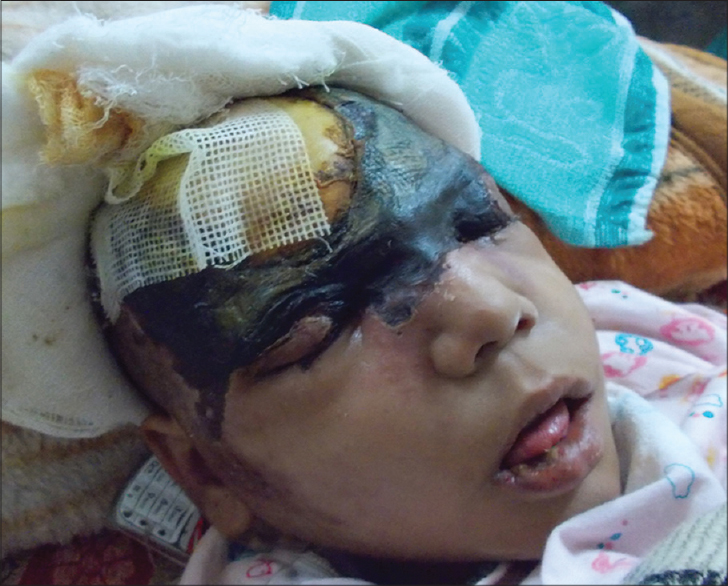 |
| Figure 1: Necrotic wound of scalp at admission |
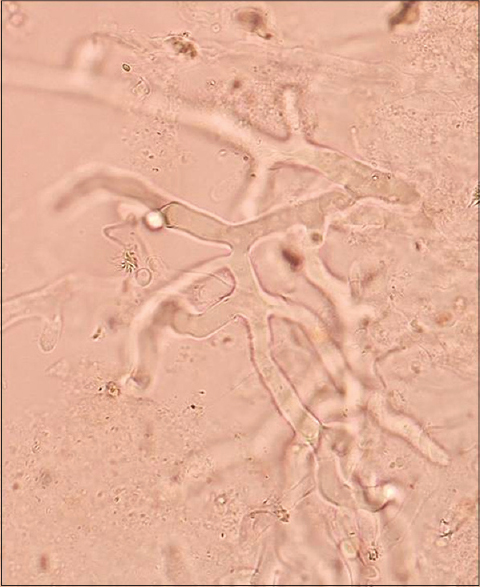 |
| Figure 2: KOH wet mount of debrided scalp tissue showing broad aseptate hyphae (10% KOH, ×400) |
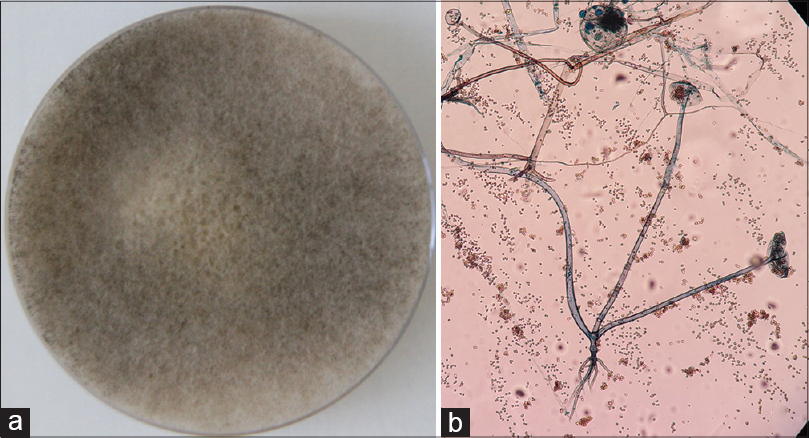 |
| Figure 3: (a) Sabouraud's dextrose agar (SDA) showing cottony growth with black spores. (b) Tease mount of mycelial growth showing features of Rhizopus oryzae (Lacto phenol cotton blue, ×100) |
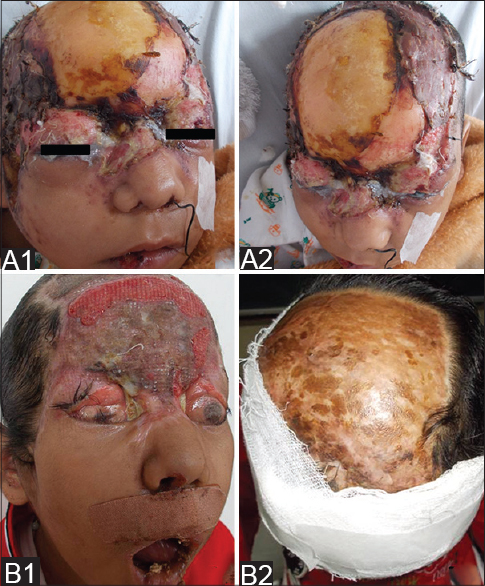 |
| Figure 4: Split skin graft after initial surgical debridement (A1 and A2). Raw areas on forehead and corneal opacity (B1) and resurfaced scalp (B2) at the time of discharge |
DISCUSSION
Mucormycosis is a rapidly progressive, potentially lethal fungal infection. [1] Rhino-orbito-cerebral and pulmonary mucormycosis are the most common presentations. Cutaneous mucormycosis accounts for 15-20% of all cases. [1] Primary disease is caused by direct implantation, while secondary disease results from dissemination from other affected sites. [2] Patients with cutaneous mucormycosis usually have underlying risk factors such as uncontrolled diabetes mellitus, ketoacidosis, hematological malignancies, or transplantation. [1],[2] However, some cases have been reported in immunocompetent individuals where trauma is the only predisposing factor. [10],[11] Primary cutaneous mucormycosis usually develops as a result of loss of skin integrity. [2] The present case had only localized disease without any evidence of dissemination. Though the extremities are more commonly involved in cutaneous mucormycosis, our case had involvement of the scalp and eyelids. Cutaneous mucormycosis of the head and neck region accounts for 14% of all cases, and scalp involvement has been documented in adults. [6],[12] We found one previously reported case of pediatric scalp mucormycosis in a severely dehydrated malnourished child. The lesion began as a black eschar at the site of an intravenous catheter on the scalp. The infection was progressive and fatal. Though aseptate hyphae suggesting mucormycosis were noted in the post-mortem histopathology samples, the exact etiological agent could not be ascertained as it was not subjected to culture. [3]
Visual impairment is an early manifestation of rhino-orbito-cerebral mucormycosis, usually caused by orbital and optic nerve compression. [2] However, in our case, visual impairment was due to inadequate lid closure and subsequent exposure keratitis, without any fungal invasion of deeper structures.
Cutaneous mucormycosis is commonly caused by Apophysomyces elegans followed by Saksenaea spp, Rhizopus spp, Absidia corymbifera, Apophysomyces variabilis and Syncephalastrum spp. [13],[14] In our case, Rhizopus oryzae was the etiological agent, as has been reported earlier in some cases. [12],[14]
A high index of suspicion, early diagnosis and aggressive management are critical for salvaging patients of cutaneous mucormycosis. [2],[13],[15] A necrotic lesion in an immunocompromised host should raise the suspicion of mucormycosis. A patient with a necrotic wound following trauma should be investigated for mucormycosis, irrespective of his/her immune status, especially if the wound continues to expand despite broad-spectrum antibiotics. Direct microscopy and culture can confirm the diagnosis, however, cultures are negative in nearly 50% of cases. [13] Therefore, molecular techniques like polymerase chain reaction followed by DNA sequencing from the clinical sample may supplement conventional methods in providing rapid diagnosis and accurate identification of mucormycetes. [15]
Extensive surgical debridement in conjunction with systemic antifungals is the mainstay of treatment. [13],[16] In the present case, surgical debridement was followed by regular monitoring for recurrence. Cutaneous mucormycosis has a better prognosis than other clinical forms of mucormycosis due to easy accessibility of lesion, although mortality still remains high (31%). [16] Early diagnosis and timely antifungal therapy prevented disease dissemination and led to healing of the lesion, though she was rendered visually impaired.
| 1. |
Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54:S23-34.
[Google Scholar]
|
| 2. |
Skiada A, Rigopoulos D, Larios G, Petrikkos G, Katsambas A. Global epidemiology of cutaneous zygomycosis. Clin Dermatol 2012;30:628-32.
[Google Scholar]
|
| 3. |
Koklu E, Akcakus M, Torun YA, Tulpar S, Tasdemir A. Primary gangrenous cutaneous mucormycosis of the scalp in a child. Pediatr Emerg Care 2008;24:102-4.
[Google Scholar]
|
| 4. |
Dave SP, Vivero RJ, Roy S. Facial cutaneous mucormycosis in a fullterm infant. Arch Otolaryngol Head Neck Surg 2008;134:206-9.
[Google Scholar]
|
| 5. |
Chakravarti A, Bhargava R, Bhattacharya S. Cutaneous mucormycosis of nose and facial region in children: A case series. Int J Pediatr Otorhinolaryngol 2013;17:55-6.
[Google Scholar]
|
| 6. |
Li H, Hwang SK, Zhou C, Du J, Zhang J. Gangrenous Cutaneous Mucormycosis Caused by Rhizopus oryzae: A Case Report and Review of Primary Cutaneous Mucormycosis in China Over Past 20 Years. Mycopathologia 2013;176:123-8.
[Google Scholar]
|
| 7. |
Jackson DP, Lewis FA, Taylor GR, Boylston AW, Quirke P. Tissue extraction of DNA and RNA and analysis by the polymerase chain reaction. J Clin Pathol 1990;43:499-504.
[Google Scholar]
|
| 8. |
Schwarz P, Bretagne S, Gantier JC, Garcia-Hermoso D, Lortholary O, Dromer F, et al. Molecular identification of zygomycetes from culture and experimentally infected tissues. J Clin Microbiol 2006;44:340-9.
[Google Scholar]
|
| 9. |
Tarai B, Gupta A, Ray P, Shivaprakash MR, Chakrabarti A. Polymerase chain reaction for early diagnosis of post-operative fungal endophthalmitis. Indian J Med Res 2006;123:671-8.
[Google Scholar]
|
| 10. |
Moran SL, Strickland J, Shin AY. Upper-extremity mucormycosis infections in immunocompetent patients. J Hand Surg Am 2006;31:1201-24.
[Google Scholar]
|
| 11. |
Kaushik R. Primary Cutaneous mucormycosis in India. Indian J Surg 2012;74:468-75.
[Google Scholar]
|
| 12. |
Harman M, Ucmak D, Dal T. A rare case of mucormycosis in the scalp. Acta Med Port 2013;26:754-7.
[Google Scholar]
|
| 13. |
Skiada A, Petrikkos G. Cutaneous zygomycosis. Clin Microbiol Infect 2009;15:41-5.
[Google Scholar]
|
| 14. |
Meis JF and Chakrabarti A. Changing epidemiology of an emerging infection: Zygomycosis. Clin Microbiol Infect 2009;15:10-4.
[Google Scholar]
|
| 15. |
Bonifaz A, Tirado-Sánchez A, Calderón L, Romero-Cabello R, Kassack J, Ponce RM, et al. Mucormycosis in children: A study of 22 cases in a Mexican hospital. Mycoses 2014;57:79-84.
[Google Scholar]
|
| 16. |
Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 2014;20:5-26.
[Google Scholar]
|
Fulltext Views
7,252
PDF downloads
2,822





