Translate this page into:
Deficit in autophagy: A possible mechanism involved in melanocyte hyperfunction in melasma
Corresponding author: Prof. Hélio Amante Miot, Departamento de Dermatologia e Radioterapia, UNESP, SN.Campus de Rubião Jr., Botucatu, SP, Brazil. heliomiot@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Espósito AC, de Souza NP, Miot LD, Miot HA. Deficit in autophagy: A possible mechanism involved in melanocyte hyperfunction in melasma. Indian J Dermatol Venereol Leprol 2021;87:585-6.
Sir,
Autophagy in the skin is essential to homeostasis, antigen presentation and adaptation or cytoprotective responses to cell damage. It can be induced by starvation, hypoxia, heavy metal exposure, oxidative stress and ultraviolet radiation. In addition, autophagy decreases with age, leading to the accumulation of damaged organelles and proteins.1
Microtubule-associated protein 1A/1B-light chain 3 (LC3) is the most important marker of autophagy. LC3 is a protein similar to ubiquitin that generates LC3-I in the cytosol when it is cleaved. LC3-I gives rise to LC3-II when conjugated to phosphatidylethanolamine, and LC3-II binds to both sides of the autophagosome membrane.1
Autophagy in melanocytes and keratinocytes influences skin pigmentation, yet, its role in melasma has not been assessed till date.2 Keratinocytes from Caucasian individuals show greater autophagic activity than those from African American individuals, which contributes to the degradation of melanosomes and a less pigmented phenotype. Therefore, there is some overlap between the regulators of autophagy and melanosome biogenesis, for example, Beclin-1 and WD repeat domain phosphoinositide-interacting- protein 1.2
In melasma, the evidence of altered fibroblasts in the upper dermis and intraepidermal retention of mature melanosomes suggest that abnormalities in autophagy and cellular senescence can support the maintenance of pigmentation in melasma.3
This study makes a comparative assessment of LC3 autophagy marker in epidermal melanocytes, pendulum melanocytes, keratinocytes and upper dermis fibroblasts of the skin samples from women affected with facial melasma and its adjacent, unaffected skin.
Twenty adult women with facial melasma who had not received treatment for more than 30 days, except for the use of sunscreen, were included in the study. These women were selected at the outpatient dermatology clinic from Botucatu Medical School— Unesp (Botucatu-SP, Brazil) from June 2018 through December 2018. Consent was obtained from all patients in the study. Two skin biopsies (3 mm) were obtained from each participant; one fragment from the skin affected with facial melasma, and the other from the adjacent (less than 2 cm apart), unaffected skin. The samples were prepared for direct fluorescence (LC3B, vimentin and 4',6-diamidino-2-phenylindole staining). The LC3B antibody is able to identify both the LC3-I and LC3-II bands. Vimentin is a marker for melanocytes and is the main intermediate filament of fibroblasts.
The fluorescence intensity of LC3B in each cell group are shown in Table 1. Cytoplasmic labeling was observed in suprabasal keratinocytes, basal layer melanocytes, pendulum melanocytes and upper dermis fibroblasts. LC3B staining was 19.2% (CI95% 3.6 to 34.8%) lower in melanocytes from the basal layer in the skin with melasma than those in the adjacent, unaffected skin [Figures 1a and 1b].
| LC3B | Melasma | Control | 95% CI difference | P* |
|---|---|---|---|---|
| Suprabasal keratinocytes | 24.6 (9.9) | 26.3 (8.7) | −6.0–2.5 | 0.381 |
| Melanocytes | 33.6 (10.0) | 38.0 (10.7) | −11.7–−1.2 | 0.025 |
| Pendulous melanocytes | 35.1 (9.0) | 37.6 (14.6) | −8.8–3.8 | 0.390 |
| Upper dermis fibroblasts | 35.8 (10.5) | 38.5 (11.6) | −8.4–2.9 | 0.304 |
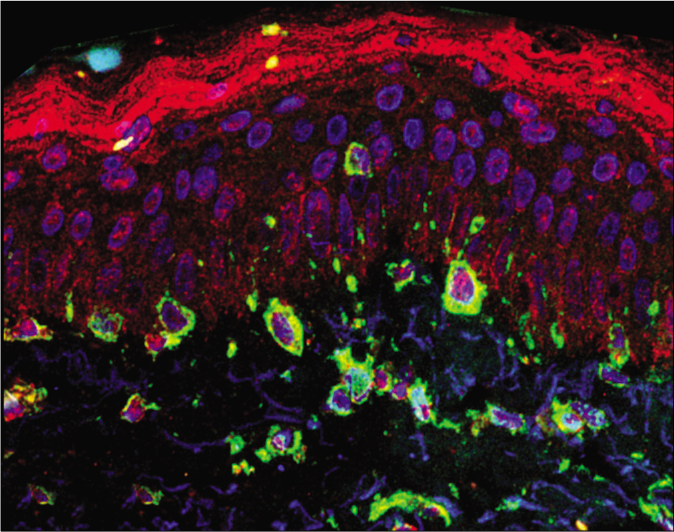
- Overlapping direct immunofluorescence images in (a) melasma— cytoplasmic labeling of melanocytes and fibroblasts (×400). LC3B—red; vimentin—green; 4',6-diamidino-2-phenylindole—blue
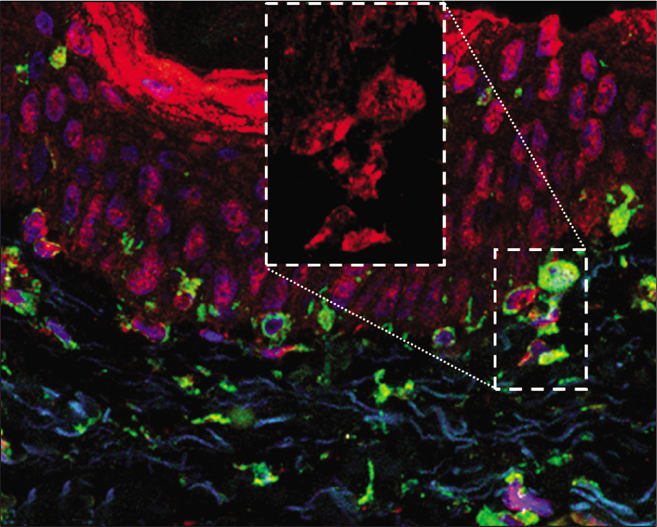
- Overlapping direct immunofluorescence images in (b) the adjacent skin—cytoplasmic labeling of melanocytes and fibroblasts (×400). LC3B— red; vimentin—green; 4',6-diamidino-2-phenylindole—blue
In melasma, both the epidermis and upper dermis are subjected to an oxidative stress microenvironment, with increased free radicals and reactive oxygen species, especially initiated by ultraviolet A radiation. Exposure to ultraviolet radiation should induce intense autophagy. Though this process occurs a few centimeters away in the unaffected, photo exposed skin, a deficit in autophagy in the skin with melasma could justify the chronic hypermelanogenesis and the retention of mature melanosomes. Conversely, the melanocytes from vitiligo and tuberous sclerosis patients show dysfunctions in autophagy that result in skin hypopigmentation.4,5
Autophagy-deficient melanocytes show a pro-inflammatory profile and release C-X-C motif chemokine ligands (CXCL1, CXCL2, CXCL10 and CXCL12) in cell culture. These ligands are associated with the pathogenesis of pigmentary disorders and with the expression of metalloproteinases 3 and 13.6 Tranexamic acid, an effective oral therapy in melasma, promotes autophagy in cultured melanoma cells by increasing the synthesis of MAPK (mitogen-activated protein kinase), extracellular signal regulatory kinase 1/2, Beclin-1, autophagy-related protein 12, LC3-I and LC3-II and by decreasing the synthesis of the mTOR (mammalian target of rapamycin) complex.7
This study has limitations related to the semiquantitative in situ exploration technique (immunofluorescence) and the investigation of only one marker among the several proteins involved in the autophagy pathway. The investigation of the molecular regulation of autophagy in melasma is warranted.
The skin with melasma has reduced expression of LC3B in basal layer melanocytes compared to the adjacent, unaffected skin. A deficit in autophagy can lead to melanocyte hyperfunction in melasma by promoting melanogenesis and regulating the physiology of melanosomes. Interventions that promote melanocyte autophagy are promising strategies for the treatment of melasma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP - Grant 2012/09233-5, 2012/05004-1), Fundo de Apoio à Dermatologia de São Paulo - Sebastião Sampaio (FUNADERSP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CnPq - Grant 401309/2016-9).
Conflicts of interest
There are no conflicts of interest.
References
- Insights into autophagy machinery in cells related to skin diseases and strategies for therapeutic modulation. Biomed Pharmacother. 2019;113:108775.
- [CrossRef] [PubMed] [Google Scholar]
- Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes. Pigment Cell Melanoma Res. 2020;33:403-15.
- [CrossRef] [PubMed] [Google Scholar]
- Prominent transepidermal melanin deposition is a distinguishing histopathological feature of melasma: A clinicopathologic study. Dermatology. 2021;237:145-7.
- [CrossRef] [PubMed] [Google Scholar]
- Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci Rep. 2017;7:42394.
- [CrossRef] [PubMed] [Google Scholar]
- Dysregulation of autophagy in melanocytes contributes to hypopigmented macules in tuberous sclerosis complex. J Dermatol Sci. 2018;89:155-64.
- [CrossRef] [PubMed] [Google Scholar]
- Autophagy deficient melanocytes display a senescence associated secretory phenotype that includes oxidized lipid mediators. Int J Biochem Cell Biol. 2016;81:375-82.
- [CrossRef] [PubMed] [Google Scholar]
- Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J Dermatol Sci. 2017;88:96-102.
- [CrossRef] [PubMed] [Google Scholar]





