Translate this page into:
Development of melanocye-keratinocyte co-culture model for controls and vitiligo to assess regulators of pigmentation and melanocytes
2 Department of Experimental Medicine and Biotechnology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Davinder Parsad
Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh-160012
India
| How to cite this article: Kumar R, Parsad D, Kanwar A, Kaul D. Development of melanocye-keratinocyte co-culture model for controls and vitiligo to assess regulators of pigmentation and melanocytes. Indian J Dermatol Venereol Leprol 2012;78:599-604 |
Abstract
Background: There is a need to develop an in vitro skin models which can be used as alternative system for research and testing pharmacological products in place of laboratory animals. Therefore to study the biology and pathophysiology of pigmentation and vitiligo, reliable in vitro skin pigmentation models are required. Aim: In this study, we used primary cultured melanocytes and keratinocytes to prepare the skin co-culture model in control and vitiligo patients. Methods: The skin grafts were taken from control and patients of vitiligo. In vitro co-culture was prepared after culturing primary melanocytes and keratinocytes. Co- cultures were treated with melanogenic stimulators and inhibitors and after that tyrosinase assay, MTT assay and melanin content assay were performed. Results: Melanocytes and keratinocytes were successfully cultured from control and vitiligo patients and after that co-culture models were prepared. After treatment of co-culture model with melanogenic stimulator we found that tyrosinase activity, cell proliferation and melanin content increased whereas after treatment with melanogenic inhibitor, tyrosinase activity, cell proliferation and melanin content decreased. We also found some differences in the control co-culture model and vitiligo co-culture model. Conclusion: We successfully constructed in vitro co-culture pigmentation model for control and vitiligo patients using primary cultured melanocytes and keratinocytes. The use of primary melanocytes and keratinocytes is more appropriate over the use of transformed cells. The only limitation of these models is that these can be used for screening small numbers of compounds.Introduction
The use of animals in research and clinical trials for testing the chemical for safety use is decreasing now days because of social and political pressure. To study the biology of pigmentation and pathophysiology of skin disease vitiligo, it is necessary to develop convenient experimental skin models that are versatile and allow translatability. Therefore research requires reliable in vitro and experimental animal models to investigate novel therapies and biologic and pathophysiologic pathways. [1] Many models have been developed, [2] but in vitro and in vivo models may show poor consistency with clinical situations. [3] In vitro human models help investigate skin pigmentation physiology in a more equivalent fashion. The advantage of in vitro models is the opportunity to analyze environmental changes, substrate and dose-response interactions in a standard fashion. [2],[4]
Melanocytes are the cells which are responsible for skin color and melanin synthesis. Melanocytes are highly responsive cells that modulate their levels of melanogenesis or proliferation according to extrinsic signals and factors derived from keratinocytes. The melanin is produced in the melanocytes and transferred to the keratinocytes. [5] Skin and hair color depends on the amount, size, and type of melanin produced by melanocytes, the subsequent transfer of the pigment to the keratinocytes, and its eventual distribution in the skin and hairs. [6],[7],[8] Melanin also protects the individual from various environmental assaults and potential cellular injury. [9],[10] Melanin prevents consequential DNA damage by effectively absorbing ultraviolet light (UV) penetrating the skin. Melanin is an effective scavenger of free radicals too. [11],[12],[13],[14],[15]
Artificial skin pigmentation models are required for the research on various pigment skin diseases and to understand the regulation of pigmentation. Also the artificial skin is required to test the chemicals and skin care products for any type of irritation, allergies, hypo-pigmentation and hyper-pigmentation. Currently the demand for artificial skin is not satisfied, and the experiments expenses are growing due to the limited amounts of artificial skin available. Many melanocyte or skin equivalent models have been used to evaluate the potential efficacy of melanogenic compounds to regulate pigmentation. Variation in results has been found in those skin equivalent models mainly because of the use of different cell lines or because of the use of keratinocytes and melanocytes alone. In vivo, melanocytes are present mainly with keratinocytes. Therefore testing of the compounds on melanocytes alone in culture does not mimic the exact conditions as found in actual skin. Keratinocytes also secretes many compounds which act on melanocytes. The melanocyte culture model alone does not reflect the effects of compounds on keratinocytes that might indirectly affect melanocyte function.
Therefore, there is a need to develop a melanocyte-keratinocyte co-culture protocol that allows testing of compounds for potential effects on pigmentation in a more physiologically relevant context. Similarly there is need for eliciting the role of various growth promoters of melanocytes in vitiligo. For this, there is a need to develop the melanocyte keratinocyte co-culture model for vitiligo subjects using vitiligo patients′ keratinocytes and melanocytes. Therefore we decided to use primary cultured melanocytes and keratinocytes to prepare the skin co-culture model in the present study.
Methods
Clinical samples
Patients with unstable vitiligo with no ongoing treatment for their disease for the previous 3 weeks were selected at the outpatient clinic of the Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. A graft (2cmX2cm) was taken on clinically active perilesional skin from five patients with vitiligo and five controls with their written informed consent and this study was approved by the ethics committee at the Postgraduate Institute of Medical Education. Control samples were obtained from the healthy control visiting skin OPD who agreed to give skin graft or Plastic Surgery department from the normal skin of patients undergoing graft surgery. Grafts were taken from lateral thigh regions of the subjects. The age range of unstable vitiligo patients and controls was 18-40 years. The mean age of patients with unstable vitiligo was 24.2 years, and of the controls was 27.6 years. Unstable vitiligo was defined as appearance of new lesions or increase in size of existing lesion over last 2 months.
Melanocyte and Keratinocyte culture
Primary culture of melanocytes and keratinocytes were established by the following procedure: Fresh biopsy specimens were obtained under aseptic conditions in phosphate buffer saline with antibiotics (penicillin and streptomycin). Skin graft was cut into small pieces (5 x 5mm) and incubated with trypsin-EDTA (0.25% trypsin and 0.02% EDTA) at 4°C overnight. Trypsin activity was required to separate the epidermis from dermis. Next day trypsin activity was neutralized by adding FBS (Fetal bovine serum) in 1:1 ratio and replaced by phosphate buffer saline solution. The epidermis was separated from the dermis with sterile forceps. Thorough pipetting was done to separate the cells and cell rich suspension was formed. The solid waste of tissue was removed and suspension was centrifuged at 1000 rpm for five minutes. Cell pellet was resuspended in serum free medium containing melanocyte basal medium with supplements and for keratinocyte culture the pellet was resuspended in keratinocyte culture media with supplements. The cell number was adjusted to 2.5 x10 4 cells per cm 2. The cultures were maintained at 37°C in a humidified, 95% air and 5% CO2 atmosphere. The medium was changed at 3-4 days intervals. The cultures were routinely examined for the contamination as well as for the outgrowth of the cells. Cells were split at confluence by trypsin treatment for 5 minutes at room temperature.
Co-culture and treatment
Cultured keratinocytes were harvested by treatment with trypsin/EDTA and seeded at 2.5 x10 5 cells/well in six-well plates. After two days, cultured melanocytes (5 x10 4 ) were collected by trypsin/EDTA and added to each well containing the keratinocytes. Co-cultures of melanocytes and keratinocytes were maintained in culture media (keratinocyte media + melanocyte media), the initial seeding ratio of keratinocytes to melanocytes being 5:1. After one day of co-culture, fresh medium containing melanocyte inhibitors and stimulators were added. Again after two days fresh medium with fresh compounds at the appropriate concentrations were added; two days after that, cultures were photographed and then harvested with trypsin/EDTA. The cells were dislodged with trypsin/EDTA; media was added to inactivate the trypsin. 100 μl cell suspension was then seeded into flat-bottom 96-well tissue culture plates for the MTT proliferation assay. The remaining cell suspension was centrifuged at 1500 g for 5 min, washed with PBS, and then solubilized in 200 μl extraction buffer at 4°C for 1 hour and various assays were then conducted [Figure - 1].
 |
| Figure 1: Flow chart showing coculture, treatment with stimulators (Latanoprost, Stem cell factor) and inhibitors (Kojic acid, Hydroquinone) and various assay performed |
Tyrosinase assay
The cells were washed with ice-cold 1XPBS. 150 μl lysis buffer (1% Triton X-100 in 0.1 M phosphate buffer) was added to each 6-well plate. Cells were scraped and transferred to a 1.5 ml tube, lysed through 3~5 times ′freezing and thawing′ cycle, in liquid nitrogen and centrifuged at 15,000 rpm for 5~10 min at 4 o C. The samples (300~500 ug/80 μl) were added to a new 96 well plate on the ice. 20 μl of 5 mM L-DOPA (L-3,4-dihydroxyphenylalanine) was added to the each well. It is then incubated at 37 o C and measured absorbance at 475 nm.
MTT assay
The MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole) assay was used to determine cell proliferation. [16] After treatment, 100 μl aliquots of cells were harvested as detailed above and plated in flat-bottom 96-well plates. Cells were allowed to attach and grow overnight at 37°C. MTT assay was performed, according to the manufacturer′s instructions. The formazan precipitates were quantitated by absorbance at 562 nm in an ELISA plate reader.
Melanin content assay
Melanin content was determined using a standard protocol. [17] Cells were lysed with 200μl 1 N NaOH and pipetted repeatedly to homogenize them. After homogenization, the cell extract was transferred into 96-well plates. Relative melanin content was determined by absorbance at 405nm in an ELISA plate reader.
Statistical analysis
Statistical analysis was performed using SPSS 14.0 (SPSS Inc., Chicago, IL, U.S.A.). The data was analyzed by ANOVA followed by Post hoc test Bonferroni. P values < 0.05 were considered as significant.
Results
Melanocytes culture
Melanocytes were successfully cultured from controls [Figure - 2]a and unstable vitiligo patients [Figure - 2]b. We found that melanocytes cultured from unstable vitiligo patients were confluent in 15 to 25 days whereas the control melanocytes took 10 to 15 days to confluency. We observed some significant differences in the morphology of melanocytes between control and unstable vitiligo patients. Melanocytes from control were normal in perinuclear region and size. On the other hand melanocytes from unstable vitiligo patients showed bigger perinuclear region. Dendrites of perilesional unstable vitiligo patients were small with clubbed ends. Dendrites of these melanocytes seem to be retracted where as the dendrites of control were normal in shape and size as mentioned in our previous paper. [18]
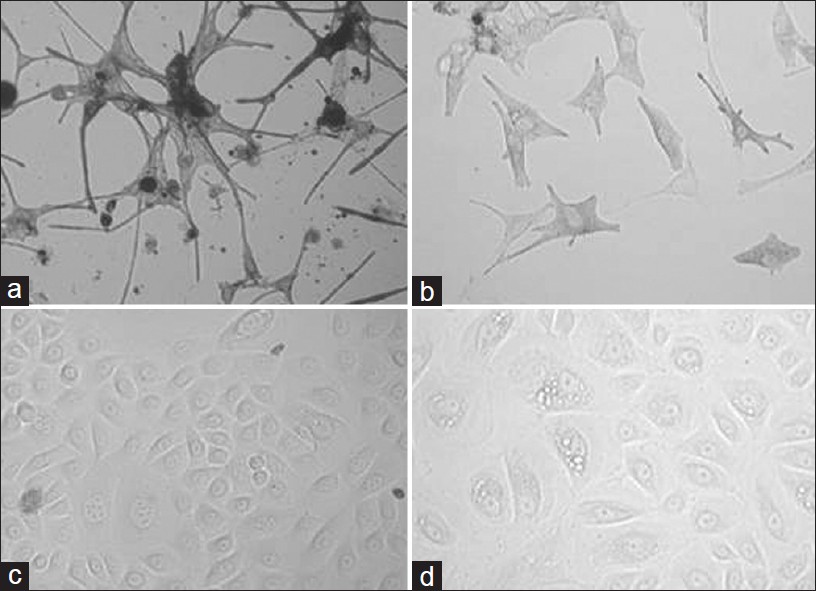 |
| Figure 2: Representative pictures of the cultured primary melanocytes from control (a) and Vitiligo vulgaris patients (b) and cultured primary keratinocytes from control (c) and Vitiligo vulgaris patients (d) |
Keratinocytes culture
We found no significant difference in the time taken by the keratinocytes to confluency between control [Figure - 2]c and vitiligo unstable patients [Figure - 2]d. The only difference we found was that the number of vacuolated keratinocytes cultured from vitiligo were more as compared to the control keratinocytes.
Co-culture of Keratinocytes and Melanocytes
After successfully culturing the melanocytes and keratinocytes we prepared co-culture model for control [Figure - 3]a and vitiligo patients [Figure - 3]b. For co-culture model we used cultured melanocytes to keratinocytes in 1:5 ratio. These co-cultures were maintained up to 6 days. First the keratinocytes were seeded in the culture plate and after two days melanocytes were added. One day later the co-cultures were treated with melanogenic compounds, which were replenished every two days.
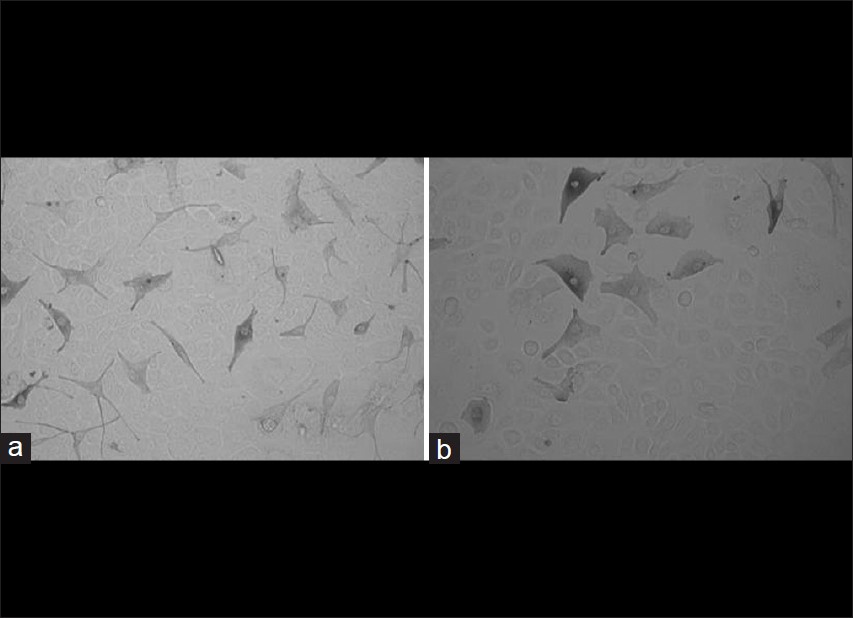 |
| Figure 3: Representative pictures of the cocultured primary melanocytes and keratinocytes from control (a) and Vitiligo vulgaris patients (b) |
Effects of Melanogenic Stimulators
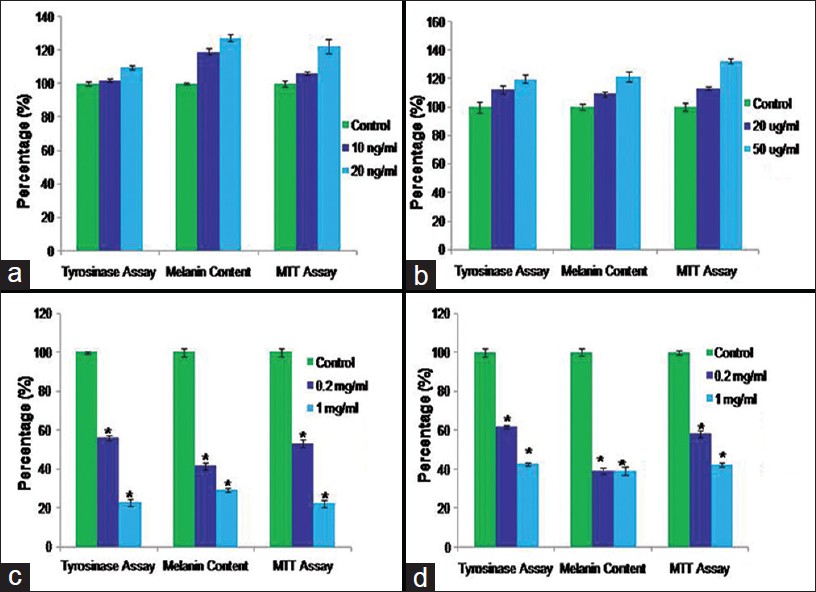
For the present research work, two melanogenic stimulators compounds stem cell factor and latanoprost which are known to stimulate melanogenesis were examined on this co-culture system and the effects on control co-culture model was compared with the effect on vitiligo co-culture model. Our results confirmed that tyrosinase activity, melanin content and proliferation increased significantly in dose dependent manner in the control co-culture model after treatment with stem cell factor and latanoprost [Figure - 4]a and b and [Figure - 5]a and b. On the other hand we found a small increase (insignificant) in the tyrosinase activity, melanin content and proliferation in vitiligo vulgaris co-culture model [Figure - 6]a and b and [Figure - 7]a and b.
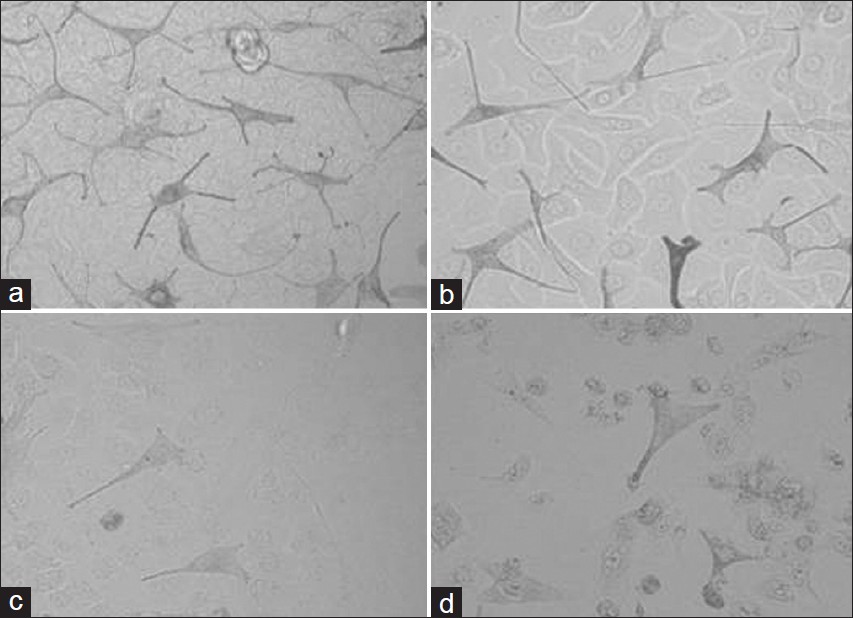 |
| Figure 4: Representative morphologies of control melanocyte-keratinocyte cocultures exposed for four days to (a) Stem cell factor (10ng/ml), (b) latanoprost (20ìg/ml), (c) Kojic acid (0.2mg/ ml) and (d) Hydroquinone (0.2mg/ml) |
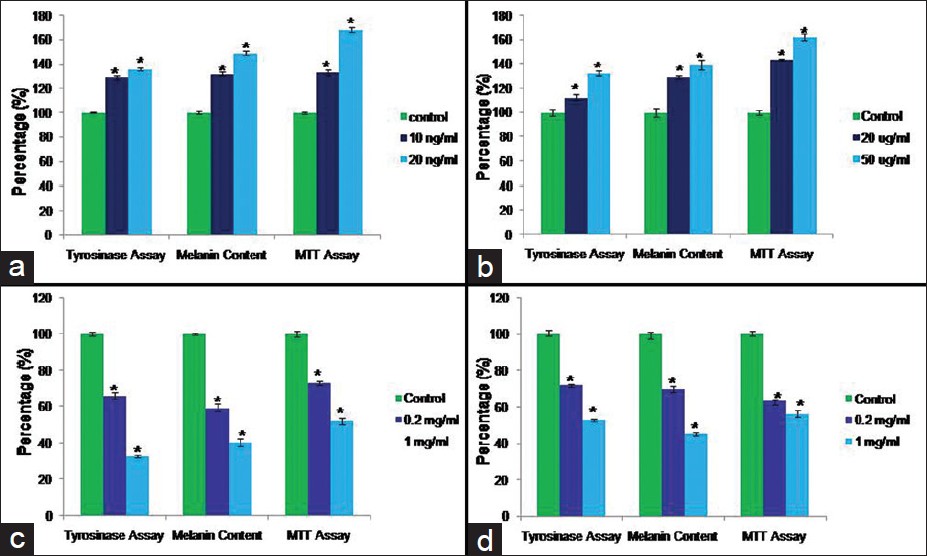 |
| Figure 5: Tyrosinase activity, Melanin content and MTT assay in control melanocytes keratinocyte coculture after treatment with the Melanogenic Stimulators (Stem cell factor (a) and latanoprost (b)) and Melanogenic Inhibitors (Kojic acid (c) and Hydroquinone (d)). Data is presented as mean ± SD. (Statistical significance is shown by *P<0.05 vs. control |
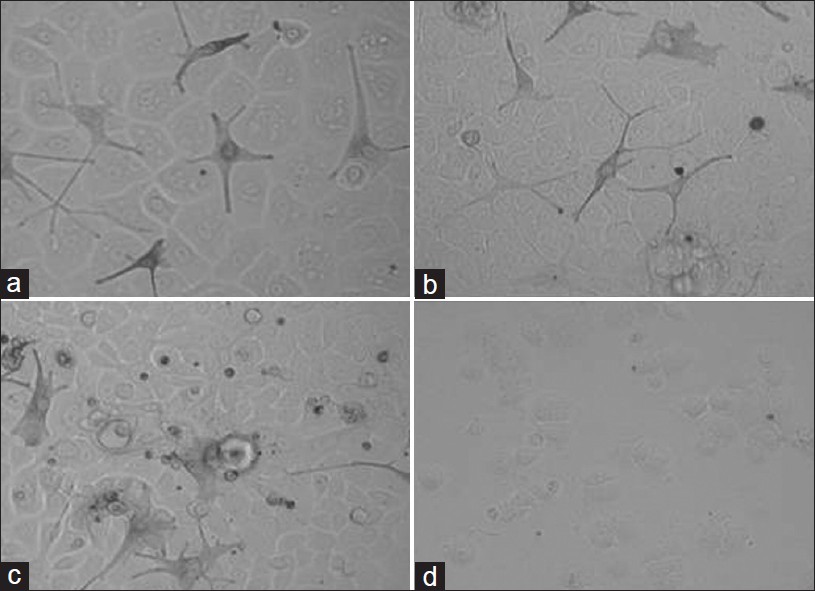 |
| Figure 6: Representative morphologies of vitiligo melanocyte-keratinocyte cocultures exposed for four days to (a) Stem cell factor (10ng/ml), (b) latanoprost (20μg/ml), (c) Kojic acid (0.2mg/ ml) and (d) Hydroquinone (0.2mg/ml) |
 |
| Figure 7: Tyrosinase activity, Melanin content and MTT assay in vitiligo melanocytes keratinocyte coculture after treatment with the Melanogenic Stimulators (Stem cell factor (a) and latanoprost (b)) and Melanogenic Inhibitors (Kojic acid (c) and Hydroquinone (d)). Data is presented as mean ± SD. (Statistical significance is shown by *P<0.05 vs. control. |
Effects of Melanogenic Inhibitors
In this study, we examined the effects of two melanogenic inhibitor compounds (Kojic acid and Hydroquinone) used in the treatment of hyperpigmentary disorders. We measured effects on tyrosinase activity, melanin content, and cell proliferation in melanocytes keratinocytes co-culture model from control and vitiligo patients. Tyrosinase activity, melanin content and proliferation decreased significantly in the co-culture model [Figure - 4]c and d
and [Figure - 5]c and d after treatment with Kojic acid and Hydroquinone. In vitiligo vulgaris co-culture model [Figure - 6]c and d and [Figure - 7]c and d, we found that the tyrosinase activity, melanin content and proliferation decreased more significantly as compared to control co-culture model.
Discussion
Many skin equivalent models have been used for evaluating the efficacy of melanogenic compounds, but each has significant shortcomings when considered as a physiologically relevant model. Pigmented melanoma cell lines have sometimes been used to assess the efficacy of melanogenic regulators, [17],[19],[20],[21] despite the fact that transformed cells are not really appropriate since they are abnormal melanocytes by definition and since their proliferative potential and disrupted intracellular signaling interfere with the normal regulation of melanin production.
Lei et al., [17] developed a melanocyte-keratinocyte co- culture protocol that allows testing of compounds for potential effects on pigmentation but they used immortalized melanocyte and keratinocyte. On the other hand we used primary cultured melanocytes and keratinocyte to developed in vitro co-culture model. We developed in vitro co-culture model for the unstable vitiligo which is a pigmentary disease and we found some significant melanocyte keratinocyte morphological differences between healthy controls and unstable vitiligo patients.
Primary melanocytes and keratinocytes would be an optimal co-culture model to test compounds. Therefore we decided to use primary cultured melanocytes and keratinocytes to prepare the skin co-culture model in the present study. We have successfully designed a melanocytes-keratinocytes co-culture model using primary cultured skin melanocytes and keratinocytes. Melanocytes and keratinocytes from vitiligo patients were morphologically different from control melanocytes and keratinocytes. The co-culture pigmentation model of vitiligo patients also shows morphological differences to that of control co-culture model, which were confirmed after treatment with pigmentation stimulators and inhibitors and performing melanin assay, MTT assay and tyrosinase assay. After treatment with melanogenic stimulators melanin content, cells proliferation and tyrosinase activity significantly increased but the increase was not significant in vitiligo co-cultured model as compared to control co-cultured model. After treatment with melanogenic inhibitors melanin content, cells proliferation and tyrosinase activity decreased which was more significant in vitiligo co-cultured model as compared to control co-cultured model.
We conclude that we successfully constructed co- culture pigmentation model for control and vitiligo patients using primary cultured melanocytes and keratinocytes. Pigmentation of the skin is because of melanin synthesis which is regulated by tyrosinase activity. These pigmentation models can be used for testing of compounds for potential effects on pigmentation in a more physiologically relevant context. Developed in vitro co-culture models can be used for screening small numbers of compounds as we are using primary cultured melanocytes and keratinocytes, as compared the models using immortalized melanocytes and keratinocytes. But the use of primary melanocytes and keratinocytes is appropriate over the use of transformed cells as the transformed cells are abnormal melanocytes and disrupted intracellular signaling interferes with the normal regulation of melanin production.
The in vitro vitiligo pigmentation model can be used to study the pathogenesis of vitiligo and effect of various compounds. We have shown this system to be a suitable model for testing known melanogenic inhibitors or stimulators.
Acknowledgments
Financial support for this research work was received from IADVL-Loreal Grant.
| 1. |
Davidson JM. Animal models for wound repair. Arch Dermatol Res 1998;290(suppl):S1-11.
[Google Scholar]
|
| 2. |
Gottrup F, Agren MS, Karlsmark T. Models for use in wound healing research: A survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen 2000;8:83-96.
[Google Scholar]
|
| 3. |
Steinstraesser L, Hirsch T, Beller J, Mittler D, Sorkin M, Pazdierny G, et al. Transient non-viral cutaneous gene delivery in burn wounds. J Gene Med 2007;9:949-55.
[Google Scholar]
|
| 4. |
Kirkpatrick CJ, Krump-Konvalinkova V, Unger RE, Bittinger F, Otto M, Peters K. Tissue response and biomaterial integration: the efficacy of in vitro methods. Biomol Eng 2002;19:211-7.
[Google Scholar]
|
| 5. |
Watt FM. The epidermal keratinocyte. Bioessays 1988;8:163-7.
[Google Scholar]
|
| 6. |
Prota G, Thomson RH. Melanin pigmentation in mammals. Endeavour 1976;35:32-8.
[Google Scholar]
|
| 7. |
Prota G. Melanin and pigmentation. In: Dolphin D, Paulson R, Abramovic O, editors. Coenzymes and Cofactors 3. New York: Wiley and Sons; 1989. p .441-66.
[Google Scholar]
|
| 8. |
Nordlund JJ, Ortonne JP. The Normal Color of Human Skin. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne JP, editors. The Pigmentary System: Physiology and Pathophysiology. New York: Oxford University Press; 1998. p. 475-88.
[Google Scholar]
|
| 9. |
Gilchrest BA. Skin aging and photoaging: An overview. J Am Acad Dermatol 1989;21:610-3.
[Google Scholar]
|
| 10. |
Bessou-Touya S, Picardo M, Maresca V, Surlève-Bazeille JE, Pain C, Taïeb A. Chimeric human epidermal reconstructs to study the role of melanocytes and keratinocytes in pigmentation and photoprotection. J Invest Dermatol 1998;111:1103-8.
[Google Scholar]
|
| 11. |
Mason HS, Ingram DJ, Allen B. The free radical property of melanins. Arch Biochem Biophys 1960;86:225-30.
[Google Scholar]
|
| 12. |
Sarna T. Properties and function of the ocular melanin-a photobiophysical view. J Photochem Photobiol B1992;12:215- 58.
[Google Scholar]
|
| 13. |
Archambault M, Yaar M, Gilchrest BA. Keratinocytes and fibroblasts in a human skin equivalent model enhance melanocyte survival and melanin synthesis after ultraviolet irradiation. J Invest Dermatol 1995;104:859-67.
[Google Scholar]
|
| 14. |
Todd C, Hewitt SD, Kempenaar J, Noz K, Thody AJ, Ponec M. Co-culture of human melanocytes and keratinocytes in a skin equivalent model: Effect of ultraviolet radiation. Arch Dermatol Res 1993;285:455-9.
[Google Scholar]
|
| 15. |
Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, et al. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: Regulation by ultraviolet B. Biochim Biophys Acta 1996;1313:130-8.
[Google Scholar]
|
| 16. |
Virador VM, Kobayashi N, Matsunaga J, Hearing VJ. A standardized protocol for assessing regulators of pigmentation. Anal Biochem 1999;270:207-19.
[Google Scholar]
|
| 17. |
Lei TC, Virador VM, Vieira WD, Hearing VJ. A melanocyte- keratinocyte coculture model to assess regulators of pigmentation in vitro. Anal Biochem 2002;305:260-8.
[Google Scholar]
|
| 18. |
Kumar R, Parsad D, Kanwar AJ. Role of apoptosis and melanocytorrhagy: A comparative study of melanocytes adhesion in stable and unstable vitiligo. Br J Dermatol 2011;164:187-91.
[Google Scholar]
|
| 19. |
Mengeaud V, Ortonne JP. Regulation of melanogenesis induced by 5-methoxypsoralen without ultraviolet light in murine melanoma cells. Pigment Cell Res 1994;7:245-54.
[Google Scholar]
|
| 20. |
Ando H, Itoh A, Mishima Y, Ichihashi M. Correlation between the number of melanosomes, tyrosinase mRNA levels, and tyrosinase activity in cultured murine melanoma cells in response to various melanogenic regulatory agents. J Cell Physiol 1995;163:608-14.
[Google Scholar]
|
| 21. |
Funayama M, Arakawa H, Yamamoto R, Nishino T, Shin T, Murao S. Effects of α and β-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci Biotechnol Biochem 1995;59:143-4.
[Google Scholar]
|
Fulltext Views
6,656
PDF downloads
2,434





