Translate this page into:
Differential expression of solute carrier family 11a member 1 and inducible nitric oxide synthase 2 in skin biopsies from leprosy patients
2 Department of Physiology, University Center of Health Sciences, Dr. Jose Barba Rubio SSJ, Guadalajara, Jalisco; Immunology Department, Instituto Dermatologico de Jalisco, Dr. Jose Barba Rubio SSJ, Guadalajara, Jalisco, México
3 Immunology Department, Instituto Dermatologico de Jalisco, Dr. Jose Barba Rubio SSJ, Guadalajara, Jalisco, México
4 Department of Medical and Pharmaceutical Biotechnology, CIATEJ, Guadalajara, Jalisco, México
5 Department of Molecular Biology, Research Institute of Biomedical Science, University Center of Health Sciences, Guadalajara University, México
Correspondence Address:
Mary Fafutis-Morris
Departamento de Fisiolog�a, Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara, Sierra Mojada No. 950, Colonia Independencia, 44340 Guadalajara, Jalisco
México
| How to cite this article: Pereira-Su�rez AL, Alvarado-Navarro A, Barrietos-Garc�a JG, Estrada-Ch�vez C, Mu�oz-Valle JF, Fafutis-Morris M. Differential expression of solute carrier family 11a member 1 and inducible nitric oxide synthase 2 in skin biopsies from leprosy patients. Indian J Dermatol Venereol Leprol 2015;81:594-599 |
Abstract
Background: Leprosy is a chronic granulomatous infection caused by Mycobacterium leprae, an intracellular parasite that resides within macrophages and cannot be eliminated effectively. Solute carrier family 11a member 1 (Slc11a1) and inducible nitric oxide synthase (iNOS), both expressed in macrophages, play major roles in host defense against several intracellular pathogens. However, the roles of these molecules in natural infection with M. leprae remain unknown. Objective: We aimed to investigate the expression of Slc11a1 and iNOS in macrophages (CD68+ cells) infiltrating skin lesions in leprosy. Methods: Skin biopsies from 48 Mexican patients of leprosy [(33 lepromatous (LL), 15 tuberculoid (TT)] and from 10 healthy controls, were subjected to immunohistochemistry to determine expression of CD68, Slc11a1 and iNOS. Results: We found a high expression of Slc11a1 and iNOS in most lepromatous leprosy samples. In tuberculoid leprosy samples, Slc11a1 expression was moderate or low, and that of iNOS was almost always low. In addition, Slc11a1 and iNOS expression levels were positively associated with bacillary loads in lepromatous leprosy lesions (P = 0.05). Conclusions: These observations suggest that M. leprae infection promotes the expression of Slc11a1 and iNOS in macrophages and that lepromatous leprosy can occur despite this response.Introduction
Leprosy is a chronic infection of skin and peripheral nerves due to Mycobacterium leprae. Although its prevalence has decreased, the incidence remains relatively static,[1] and it is still an important public health problem in some countries around the world. Based on immunological, histopathological and microbiological parameters, leprosy has been classified as lepromatous leprosy, borderline lepromatous, borderline-borderline, borderline tuberculoid and tuberculoid leprosy.[2] At opposite ends of the disease spectrum, tuberculoid leprosy typifies a resistant response that restricts growth of the pathogen while lepromatous leprosy represents susceptibility to disseminated infection. These presentations correlate respectively with levels of cell-mediated and humoral immune responses against M. leprae.[3]
Although infiltration by macrophages is prominent across the disease spectrum, they are well-differentiated and rarely contain bacteria in tuberculoid leprosy, whereas lepromatous leprosy is characterized by abundant intracellular bacilli and foamy macrophage differentiation, the latter due to the accumulation of host- and pathogen-derived lipids.[4]
In the phagosome, pathogens are normally subjected to massive attack by reactive oxygen and nitrogen intermediates.[5] This is achieved by two macrophage-specific enzymatic pathways viz., phagocyte oxidase and inducible nitric oxide synthase (iNOS).[6],[7] Macrophage-mediated innate resistance to intracellular pathogens like Mycobacterium is affected by a dominant gene named the natural resistance-associated macrophage protein 1 (NRAMP1), known as solute carrier family 11a member 1 (Slc11a1) or Ity/Lsh/Bcg. The function of Slc11a1 as an iron (Fe 2+) and divalent cation transporter is still controversial. It either increases transphagosomal Fe 2+ transport, catalyzing the Haber–Weiss/Fenton reaction to generate the highly toxic hydroxyl radical essential for macrophage bactericidal activity, or it reduces the intraphagosomal availability of Fe 2+ and other divalent cations which are critical for the invading pathogens to survive phagosomal damage.[8]
We have in previous studies demonstrated a high expression of NRAMP1 in granulomas from cattlenaturally infected with M. bovis. We also found co-expression of NRAMP1 and iNOS in macrophages from granulomatous lesions in bovines naturally infected with Mycobacterium avium subspecies paratuberculosis (Johne's disease).[9],[10] In the present study we investigated the co-expression of Slc11a1 and iNOS in infiltrating macrophages (CD68+ cells) in skin biopsies from leprosy patients. CD68, associated with lysosomal glycoproteins, is a specific marker for human monocytes and macrophages.[11]
Methods
The study was carried out at the Dermatology Institute of Jalisco, “Dr. José Barba Rubio,” Guadalajara Jalisco México, from 2010 to 2013, and approved by the Research Ethics Committee in Biomedical Sciences (Guadalajara University, Guadalajara, Jalisco, Mexico and the Dermatology Institute of Jalisco “Dr. José Barba Rubio,” Guadalajara Jalisco México). The study was in accordance with the guidelines of the Mexican official standards (Norma Official Mexicana) and the World Medical Association Declaration of Helsinki.[12]
Patients and tissue samples
Tissues samples were obtained from 48 patients with leprosy: 33 cases of lepromatous leprosy and 15 of tuberculoid leprosy, categorized in accordance with the Ridley and Jopling classification, and 10 healthy controls. Patients had not received treatment when biopsies were taken for this study and the time of duration of the disease was unknown in all patients.
Skin samples were fixed in 4% formalin and embedded in paraffin. Sections obtained were stained with hematoxylin/eosin and Kinyoun stains, the latter to detect acid-fast bacilli (bacilli staining red and cells, blue). Histopathologic characterization, carried out by pathologists experienced in evaluating leprosy, was also based on the criteria of Ridley and Jopling.[13]
Immunohistochemical staining
Serial sections were processed to detect Slc11a1, iNOS and CD68 by immunohistochemistry. Sections were deparaffinized with successive immersions in 100% xylene, 100% ethanol, 96% ethanol and 70% ethanol for 10, 10, 5 and 5 minutes respectively.[14] Endogenous peroxidase activity was inactivated by incubating with peroxidase blocking reagent (S2001, DAKO) for 10 minutes. The slides were treated to 10 mM citrate buffer (pH 6) and heated to 121°C for 15 minutes. Next, blockade with 50 µl of 1% Bovine Serum Albumin (BSA) (Sigma, USA) in Tris-buffered saline with Tween 20 (TBST) buffer (50 mM, Tris-HCl, 150 mM NaCl, 0.1% Tween 20) for 5 minutes was carried out at room temperature. Sections were then incubated overnight with 40 µl of anti-NRAMP1 (Slc11a1) (Santa Cruz Biotechnology Inc., Cat num. sc-20113) diluted 1:100 (200 µg/µl), anti-iNOS2 (BD Transduction Lab., Cat num. 610332), diluted 1:100 (250 µg/µl), or anti-CD68 (Dako Cytomation, Denmark A/S, M0876) diluted 1:75 (32 mg/L), all diluted in TBST buffer. Subsequently, sections were washed with TBST and each incubated with one drop of secondary antibody conjugated with horseradish peroxidase (K4061, DAKO) for 60 minutes at room temperature. After washing, the sections were each incubated with one drop of chromogenic diaminobenzidine substrate (K3468, DAKO) for 15 minutes at room temperature. They were then counterstained with Mayer's hematoxylin and mounted with an aqueous-based mounting medium (Vectamount AQ). All sections were developed in parallel with a negative control, where the primary antibody was omitted.
Microscopic analysis
Specimens were analyzed with an optical microscope (Carl Zeiss, Göttingen, Germany) with the ×10, ×20, ×40 and ×100 objectives. Images from the ×40 objectives were captured with an AxioCam MRm digital camera (Carl Zeiss) and recorded with AxioVs40 V 4.8.2.0 software (Carl Zeiss). Fluorescence intensities were rated on an arbitrary scale as follows: low (5–15 cells), moderate (15–50 cells) and high (>50 cells).
Statistics
P values were calculated based on a one-way Chi-square (χ2) test when patients' biopsies were compared with controls, and values ≤0.05 were considered significant.
Results
The average age of patients was 58 ± 16 years, 41% were females and 49% males and there were 33 lepromatous leprosy (LL) patients and 15 tuberculoid leprosy (TT) patients. Biopsies were classified according to the histology [Table - 1]. Since 87% of leprosy patients in Mexico are at the lepromatous pole,[15] the number of tuberculoid leprosy samples analyzed in this study was relatively low.

Characteristic lesions of leprosy were observed in hematoxylin and eosin–stained slides (not shown). Lymphocytes, epithelioid macrophages and giant cells of the Langhans type were observed throughout the granulomas in tuberculoid leprosy, and foamy macrophages were found in lepromatous leprosy. Kinyoun staining revealed few AFB in tuberculoid leprosy and many bacilli in lepromatous leprosy (not shown). CD68 was expressed by macrophages and macrophage-derived cells including foam cells in lepromatous leprosy [Figure - 1]c and [Figure - 1]d, and by giant cells and epithelioid cells in tuberculoid leprosy [Figure - 2]c and [Figure - 2]d. The CD68 immunoreactivity was moderate to strong in both polar forms of leprosy, while no signal was detected in the healthy subjects' skin biopsies.
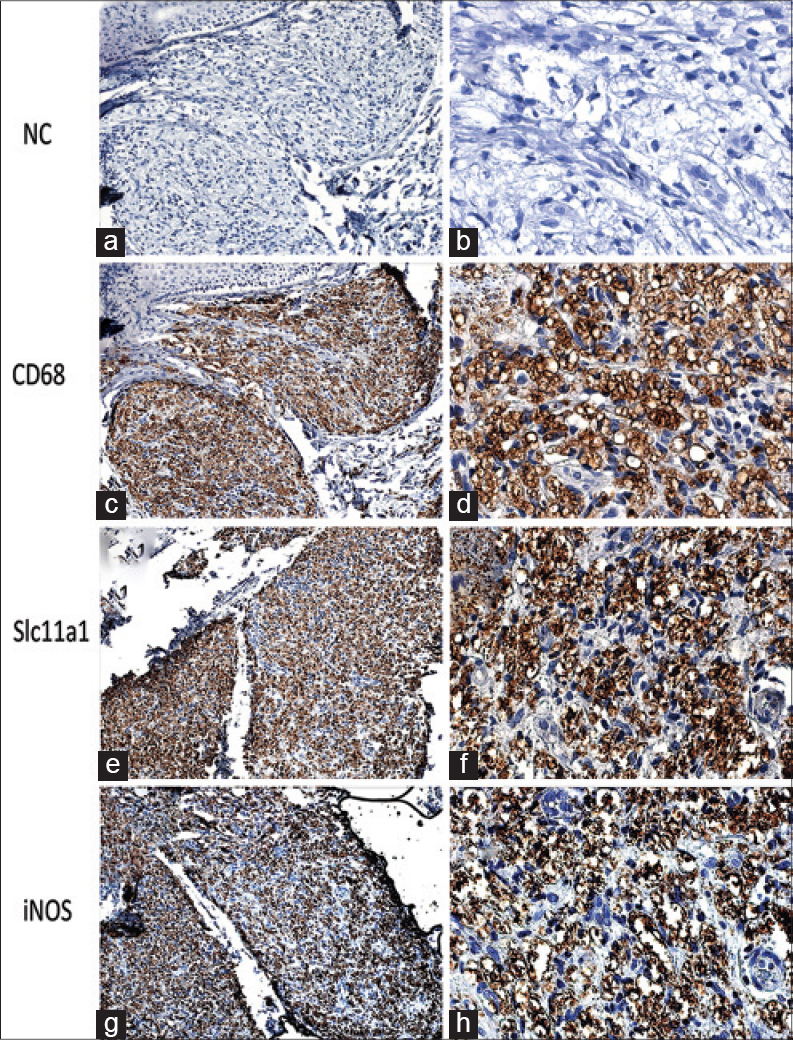 |
| Figure 1: Expression of CD68 (c and d), (e and f) Slc11a1, and (g and h) iNOS in skin biopsies of lepromatous leprosy; (a and b) negative control with primary antibodies omitted. Immunoperoxidase stain, ×200 (a, c, e, g), ×400 (b, d, f, h) |
 |
| Figure 2: Expression of CD68 (c and d), (e and f) Slc11a1, and (g and h) iNOS in skin biopsies of tuberculoid leprosy; (a and b) negative control with primary antibodies omitted. Immunoperoxidase stain, ×200 (a, c, e, g), ×400 (b, d, f, h) |
A semi-quantitative estimation of the immunolabeling of cells with the anti-Slc11a1 or anti-iNOS antibodies is presented in [Table - 2]. Both were detected in all leprosy biopsies with varying intensities. The percentage of immunolabeled cells was classified as low: (5–15%), moderate: (16–50%), or high: more than 50%. The number of Slc11a1-labeled foamy macrophages and the intensity of labeling was very high in most of the lepromatous leprosy samples [Figure - 1]e and [Figure - 2]f. However, immunolabeling of Slc11a1 was moderate and low in 11 and 3 of the tuberculoid leprosy samples respectively [Figure - 2]e and [Figure - 2]f, and high in only one. Immunolabeling of iNOS too followed a similar pattern, being high in 29 of the lepromatous leprosy cases, moderate in 3 and low in only 1 case. In contrast, the expression of iNOS was very low in 11 of the tuberculoid leprosy samples and moderate in the other 4 [Figure - 2]. There was a direct relationship between the intensity of Slc11a1 and iNOS immunostaining and the type of leprosy [Table - 2]; P = 0.05].

Biopsies from the 10 healthy controls exhibited little or no immunostaining [Figure - 3].
 |
| Figure 3: Expression of CD68, Slc11a1, and iNOS in biopsies of healthy skin. Immunoperoxidase stain, ×200 |
Discussion
A recent study showed high expression of iNOS in 78% of leprosy biopsies,[16] a finding which may be associated with bactericidal or bacteriostatic capacity. In the present study, we found that the bacterial load was directly related to the intensity of immunostaining for iNOS and Slc11a1, but not to that for CD68.
Different subpopulations of macrophages display different phenotypes with some having a high capacity for fighting other infections, but low antimycobacterial activity.[17],[18] Patients of tuberculoid and lepromatous leprosy were found to have macrophages that were unable to lyse live M. leprae.[19] A previous analysis of macrophages infiltrating leprosy lesions had also shown altered regulation of phagocytosis and antimicrobial responses.[20]
Tests of the M. leprae-killing ability of resting and gamma interferon-activated macrophages from normal subjects and patients with leprosy suggested that the innate ability of macrophages might be of greater importance in conferring protection against M. leprae infection than lymphocyte-mediated activation.[21] It is likely that infiltrating macrophages in lepromatous leprosy are defective in processing M. leprae and antigen presentation.[22] These defects may in turn reduce the secretion of interferon-gamma and tumor necrosis factor-α and favor a Th2 immune profile as the disease progresses.[23] Host genetic factors might be important in controlling mycobacterial infections regardless of the bacterial genotype or strain.[24] Though studied a decade ago, no linkage between leprosy susceptibility and the human Slc11a1 gene was found in some multi-case leprosy families.[25] More recently, tuberculoid, but not lepromatous leprosy, was reported to be associated with the Slc11a1 locus.[26]
Our results suggest that M. leprae infection might upregulate the expression of Slc11a1 and iNOS in lesional foamy macrophages in lepromatous leprosy. This could be mediated by cytokines in the lesional microenvironment. It could also be that iNOS expression is upregulated by Slc11a1. It has been reported that murine macrophages carrying functional Slclla1 display increased nitric oxide synthesis that is abrogated by iNOS inhibitors while iNOS production is markedly diminished in mice with mutated Slc11a1.[27]
In view of our findings, NRAMP1 and iNOS could be considered part of the arsenal of antimycobacterial molecules expressed in leprosy lesions. However, upregulated Slc11a1 and iNOS expression may not be sufficient to eliminate the pathogen in lepromatous leprosy. Other important mechanisms in signaling, phagosome formation and maturation contributing to the bactericidal activity of macrophages were not included in this work. Further studies including several cell markers and examining both skin biopsies and serum samples are needed. Genetic analyses looking at associations between allelic variants of genes codifying Slc11a1 and iNOS and different types of leprosy may help to elucidate their role further.
Conclusion
Our observations suggest that M. leprae infection promotes the expression of Slc11a1 and iNOS by human macrophages. Their expression being particularly strong in foamy cells suggests that these molecules may be involved in the host defense against M. leprae.
Acknowledgments
We would like to thank Hernández Torres M., MD, Pathologist, for her skilful technical assistance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Global leprosy situation, 2012. Wkly Epidemiol Rec 2012;87:317-28.
[Google Scholar]
|
| 2. |
Parkash O. Classification of leprosy into multibacillary and paucibacillary groups: An analysis. FEMS Immunol Med Microbiol 2009;55:1-5.
[Google Scholar]
|
| 3. |
Modlin RL. The innate immune response in leprosy. Curr Opin Immunol 2010;22:48-54.
[Google Scholar]
|
| 4. |
Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, et al. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest 2008;118:2917-28.
[Google Scholar]
|
| 5. |
Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 2000;97:8841-8.
[Google Scholar]
|
| 6. |
Wyllie S, Seu P, Goss JA. The natural resistance-associated macrophage protein 1 Slc11a1 (formerly Nramp1) and iron metabolism in macrophages. Microbes Infect 2002;4:351-9.
[Google Scholar]
|
| 7. |
Barton CH, Biggs TE, Baker ST, Bowen H, Atkinson PG. Nramp1: A link between intracellular iron transport and innate resistance to intracellular pathogens. J Leukoc Biol 1999;66:757-62.
[Google Scholar]
|
| 8. |
Blackwell JM, Searle S, Mohamed H, White JK. Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story. Immunol Lett 2003;85:197-203.
[Google Scholar]
|
| 9. |
Pereira-Suárez AL, Estrada-Chávez C, Arriaga-Díaz C, Espinosa-Cueto P, Mancilla R. Coexpression of NRAMP1, iNOS, and nitrotyrosine in bovine tuberculosis. Vet Pathol 2006;43:709-17.
[Google Scholar]
|
| 10. |
Delgado F, Estrada-Chávez C, Romano M, Paolicchi F, Blanco-Viera F, Capellino F, et al. Expression of NRAMP1 and iNOS in Mycobacterium avium subsp. paratuberculosis naturally infected cattle. Comp Immunol Microbiol Infect Dis 2010;33:389-400.
[Google Scholar]
|
| 11. |
Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993;81:1607-13.
[Google Scholar]
|
| 12. |
World Medical Association. Special communication. Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. 64th WMA General Assembly, Fortaleza, Brazil, October 2013. Available from: http://jama.jamanetwork.com/on. [Last accessed on 2015 Aug 15].
[Google Scholar]
|
| 13. |
Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev 1962;33:119-28.
[Google Scholar]
|
| 14. |
Wiley EL, Mulhollan TJ, Beck B, Tyndall JA, Freeman RG. Polyclonal antibodies raised against Bacillus Calmette-Guerin, Mycobacterium duvalii, and Mycobacterium paratuberculosis used to detect mycobacteria in tissue with the use of immunohistochemical techniques. Am J Clin Pathol 1990;94:307-12.
[Google Scholar]
|
| 15. |
Larrea MR, Carreño MC, Fine PE. Patterns and trends of leprosy in Mexico: 1989-2009. Lepr Rev 2012:83;184-94.
[Google Scholar]
|
| 16. |
Lockwood DN, Suneetha L, Sagili KD, Chaduvula MV, Mohammed I, van Brakel W, et al. Cytokine and protein markers of leprosy reactions in skin and nerves: Baseline results for the North Indian INFIR cohort. PLoS Negl Trop Dis 2011;5:e1327.
[Google Scholar]
|
| 17. |
Veliath AJ, Bedi BM, Balasubrahmanyan M. Macrophage culture from untreated leprosy cases. Lepr India 1980;52:203-8.
[Google Scholar]
|
| 18. |
Parmaswaran M, Girdhar BK, Deo MG, Kandhari KC, Bhutani LK. Macrophage function in leprosy. Int J Lepr Other Mycobact Dis 1976;44:340-5.
[Google Scholar]
|
| 19. |
Montoya D, Cruz D, Teles RM, Lee DJ, Ochoa MT, Krutzik SR, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe 2009;6:343-53.
[Google Scholar]
|
| 20. |
Salgame PR, Birdi TJ, Lad SJ, Mahadevan PR, Antia NH. Mechanism of immunosuppression in leprosy – macrophage membrane alterations. J Clin Lab Immunol 1984;14:145-9.
[Google Scholar]
|
| 21. |
Desai SD, Birdi TJ, Antia NH. Correlation between macrophage activation and bactericidal function and Mycobacterium leprae antigen presentation in macrophages of leprosy patients and normal individuals. Infect Immun 1989;57:1311-7.
[Google Scholar]
|
| 22. |
Birdi TJ, Mistry NF, Mahadevan PR, Antia NH. Antigen specific macrophage-lymphocyte interaction in lepromatous leprosy. J Clin Lab Immunol 1984;13:189-94.
[Google Scholar]
|
| 23. |
Hussain R, Kifayet A, Dojki M, Dockrell HM. Selective correlation of interferon-gamma, tumour necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor with immunoglobulin G1 and immunoglobulin G3 subclass antibody in leprosy. Immunology 1999;98:238-43.
[Google Scholar]
|
| 24. |
van Crevel R, Parwati I, Sahiratmadja E, Marzuki S, Ottenhoff TH, Netea MG, et al. Infection with Mycobacterium tuberculosis Beijing genotype strains is associated with polymorphisms in SLC11A1/NRAMP1 in Indonesian patients with tuberculosis. J Infect Dis 2009;200:1671-4.
[Google Scholar]
|
| 25. |
Roger M, Levee G, Chanteau S, Gicquel B, Schurr E. No evidence for linkage between leprosy susceptibility and the human natural resistance-associated macrophage protein 1 (NRAMP1) gene in French Polynesia. Int J Lepr Other Mycobact Dis 1997;65:197-202.
[Google Scholar]
|
| 26. |
Sunderkötter CH, Tomimori-Yamashita J, Nix V, Maeda SM, Sindrilaru A, Mariano M, et al. High expression of myeloid-related proteins 8 and 14 characterizes an inflammatorily active but ineffective response of macrophages during leprosy. Immunology 2004;111:472-80.
[Google Scholar]
|
| 27. |
Arias M, Rojas M, Zabaleta J, Rodríguez JI, París SC, Barrera LF, et al. Inhibition of virulent Mycobacterium tuberculosis by Bcg (r) and Bcg (s) macrophages correlates with nitric oxide production. J Infect Dis 1997;176:1552-8. Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993;81:1607-13.
[Google Scholar]
|
Fulltext Views
3,009
PDF downloads
2,047





