Translate this page into:
Disseminated molluscum contagiosum following chemotherapy: A therapeutic challenge
2 Department of Dermatology and Venereology, Government Medical College, Kozhikode, Kerala, India
3 Department of Surgery, Government Medical College, Kozhikode, Kerala, India
4 Department of Ophthalmology, Government Medical College, Kozhikode, Kerala, India
Correspondence Address:
Sarita Sasidharanpillai
“Rohini,” Girish Nagar, Nallalom PO, Kozhikode - 673 027, Kerala
India
| How to cite this article: Ajithkumar VT, Sasidharanpillai S, Muhammed K, Sreejayan MP, Simin M, Ashraf F, John N, Dhanyasree T. Disseminated molluscum contagiosum following chemotherapy: A therapeutic challenge. Indian J Dermatol Venereol Leprol 2017;83:516 |
Sir,
Disseminated molluscum contagiosum is a marker of immunodeficiency. We report a child with extensive molluscum contagiosum who required extraction under general anesthesia to control widespread lesions that developed following chemotherapy.
A 9-year-old male child, who completed the modified pediatric Berlin–Frankfurt–Munster protocol for acute lymphatic leukemia (high risk) two months previously, was referred to us for the management of histologically-confirmed disseminated molluscum contagiosum. He had numerous lesions on the face [Figure - 1], buttocks, thighs and groins, along with several scattered lesions on the abdomen and limbs. Most of the lesions were inflamed and many showed evidence of secondary infection.
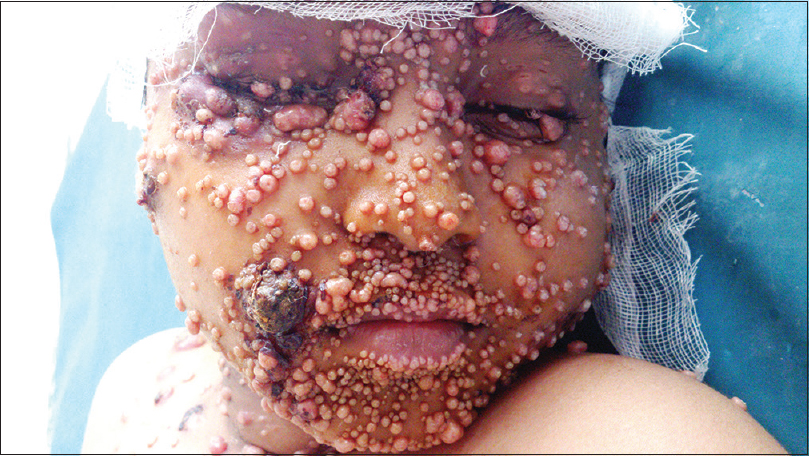 |
| Figure 1: Extensive lesions of molluscum contagiosum on the face of a child following chemotherapy |
The molluscum lesions had first appeared during chemotherapy and were resistant to multiple modes of therapy including cryotherapy and topical application (six sittings) of 35% trichloroacetic acid and 10% potassium hydroxide. Extraction with a sterile needle was effective, but recurrence was the rule. After successful completion of chemotherapy, previously asymptomatic lesions became painful and inflamed and the child was unable to tolerate further extraction, which led to the dissemination of lesions to the present state. Investigations revealed polymorphonuclear leukocytosis, sterile blood culture and a negative serology for human immunodeficiency virus (HIV) infection. He received parenteral ceftazidime and netilmicin, as the pus culture isolated Proteus fecalis and Escherichia coli sensitive to these drugs.
The infected molluscum lesions interfered with eye-opening, produced cosmetic disfigurement and placed the child at high risk for eye infection. The inflamed and macerated groin lesions affected the child's mobility. Topical 10% potassium hydroxide, phenol cautery and cryotherapy were ineffective. Electrocautery, extraction and carbon dioxide (CO2) laser did not help significantly since only a limited number of lesions could be treated at one time due to the pain associated with the procedure and bleeding from the lesions.
Hence, we opted for molluscum extraction under general anesthesia so as to remove all the lesions that were compromising his daily routine in a single sitting.
After explaining the added complications of secondary infection and delayed wound healing to the parents, molluscum extraction limited to the face and groin lesions was performed. Difficult mask ventilation was induced with propofol and maintained with oxygen, nitrous oxide and isoflurane for 2½ hours. Extraction of numerous lesions in a single sitting resulted in significant bleeding necessitating transfusion of two units of fresh blood after the procedure. It was decided to maintain a broad spectrum antibiotic cover (parenteral ceftazidime, metrogyl, netilmicin and linezolid) for 14 days, along with topical cleansing measures so as to prevent the complication of septicemia. He could open his eyes completely by the third post-procedure day, regained normal mobility by the end of the week and was discharged on the 14th post-procedure day on amoxicillin-clavulanic acid combination and the advice to apply 10% potassium hydroxide solution daily at bedtime on a limited number of lesions on the trunk and limbs. His review at the end of the month showed a marked reduction in the number of remaining molluscum lesions [Figure - 2]. Now, six months after the procedure, he remains asymptomatic and leads a normal life.
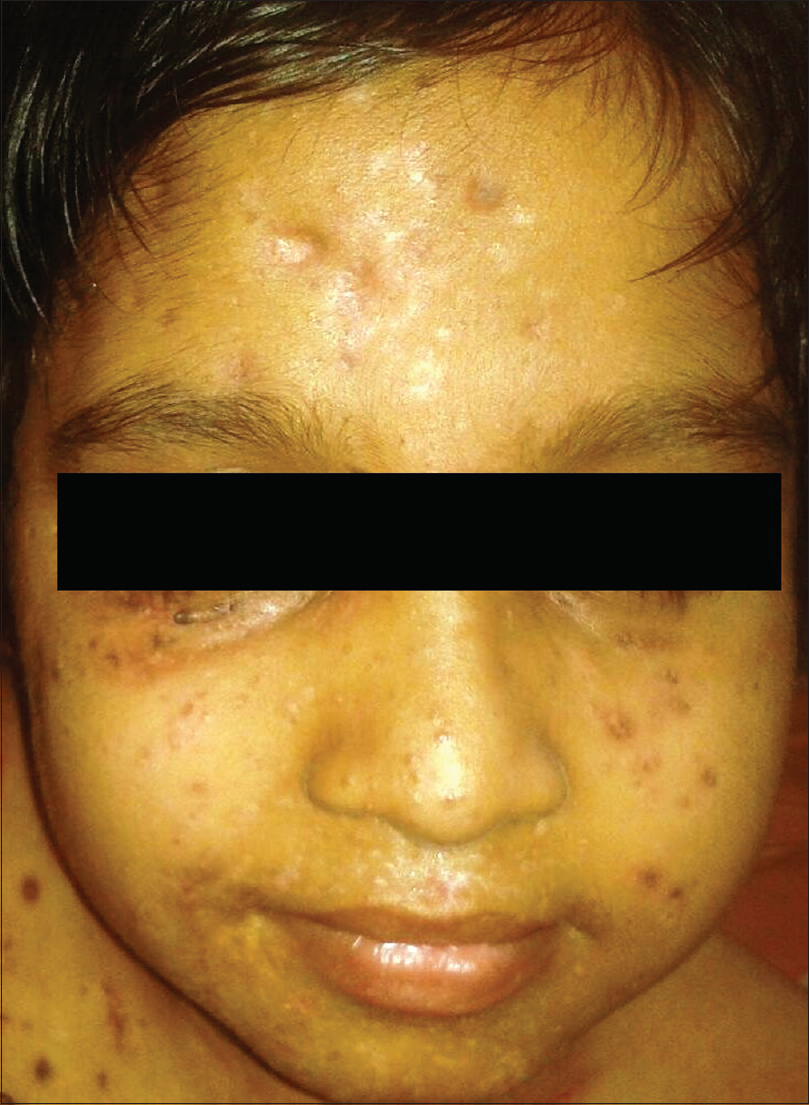 |
| Figure 2: The same child after extraction of lesions under general anesthesia |
The average duration of molluscum infection in the immunocompetent varies from 3 months up to 5 years.[1] Autoinoculation of adjoining cutaneous areas plays an important role in the persistence of infection.
The preferential involvement of face, groin and buttocks, as noted in our patient, has been cited as a feature of molluscum infection in immunodeficiency.[2],[3] The asymptomatic nature of the molluscum lesions throughout the chemotherapy period and the development of acute inflammation after completion of the treatment could be explained as due to the therapy-induced immunosuppression and subsequent immune recovery, respectively. Inflamed molluscum has been reported as a manifestation of immune reconstitution inflammatory syndrome in patients on highly active antiretroviral therapy.[4]
None of the therapeutic options found useful in disseminated molluscum such as topical imiquimod, cidofovir, cimetidine, photodynamic therapy, CO2 laser and pulse dye laser uniformly lead to immediate relief as needed in our patient.[3] Skorin and Nashreported curettage of about 20 molluscum lesions on the eyelids of a 5-year-old child under general anesthesia, but we were unable to find any previous reports of disseminated molluscum contagiosum managed by widespread extraction under general anesthesia.[5]
The better response to topical preparations, following the removal of a significant number of face and groin lesions in our case, could be attributed to the post-procedure immune stimulation and the marked reduction in lesions by the procedure.
Acknowledgment
We are grateful to Dr. K. Subin and Dr. B.N. Indu, Junior Residents, Department of Dermatology and Ophthalmology respectively, Government Medical College, Kozhikode, Dr. K.R. Radha, Additional Professor, Department of Anesthesia, Government Medical College, Kozhikode and Dr. T.M. Sheeja Rajan, Associate Professor, Department of Plastic and Reconstructive Surgery, Government Medical College, Kozhikode for their invaluable help in treating this patient. We also express our sincere gratitude to Dr. M. Ramam, Professor, Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi for his timely advice in managing this patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given his consent for images and other clinical information to be reported in the journal. The guardian understands that names and initials will not be published and due efforts will be made to conceal patient identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Tyring SK. Molluscum contagiosum: The importance of early diagnosis and treatment. Am J Obstet Gynecol 2003;189 3 Suppl:S12-6.
[Google Scholar]
|
| 2. |
Singh RV, Singh S, Pandey SS. Numerous giant mollusca contagiosa and kaposi's sarcomas with HIV disease. Indian J Dermatol Venereol Leprol 1996;62:173-4.
[Google Scholar]
|
| 3. |
Vora RV, Pilani AP, Kota RK. Extensive giant molluscum contagiosum in a HIV positive patient. J Clin Diagn Res 2015;9:WD01-2.
[Google Scholar]
|
| 4. |
Vozmediano JM, Manrique A, Petraglia S, Romero MA, Nieto I. Giant molluscum contagiosum in AIDS. Int J Dermatol 1996;35:45-7.
[Google Scholar]
|
| 5. |
Skorin L, Nash P. Five-year-old Diagnosed with Molluscum Contagiosum: The Lesions are Typically Treated with Removal. Primary Care Optometry News; October, 2015.
[Google Scholar]
|
Fulltext Views
4,030
PDF downloads
3,045





