Translate this page into:
Distal infection of the middle finger caused by Mycobacterium abscessus
2 Department of Pathology, Hospital Clínic de Barcelona, Universitat de Barcelona, Barcelona, Spain
Correspondence Address:
Pilar Iranzo-Fernandez
Dermatology Unit, Hospital Clínic de Barcelona, C/Villarroel, 170, Zip Code 08036, Barcelona
Spain
| How to cite this article: Morgado-Carrasco D, Loughlin CR, Fusta-Novell X, Garcia A, Iranzo-Fernandez P. Distal infection of the middle finger caused by Mycobacterium abscessus. Indian J Dermatol Venereol Leprol 2018;84:626-628 |
Sir,
An otherwise healthy woman in her 60 s without any past medical history or previous trauma presented to our dermatology department with a painful swelling of her right middle finger since last 4 months. She had been previously treated with oral amoxicillin-clavulanic acid, levofloxacin, rifampin and fluconazole without any appreciable response. She had also undergone two surgical debridements, including a nail extraction at the traumatology unit, due to severe tumefaction of the finger, but lesions persisted.
On physical examination, the distal part of her right middle finger was erythematous and swollen, with annular crusts in a confluent pattern [Figure - 1]. Lesions were tender on palpation. Hair, nails and mucosae were spared. Rest of her general and systemic examination was within normal limits.
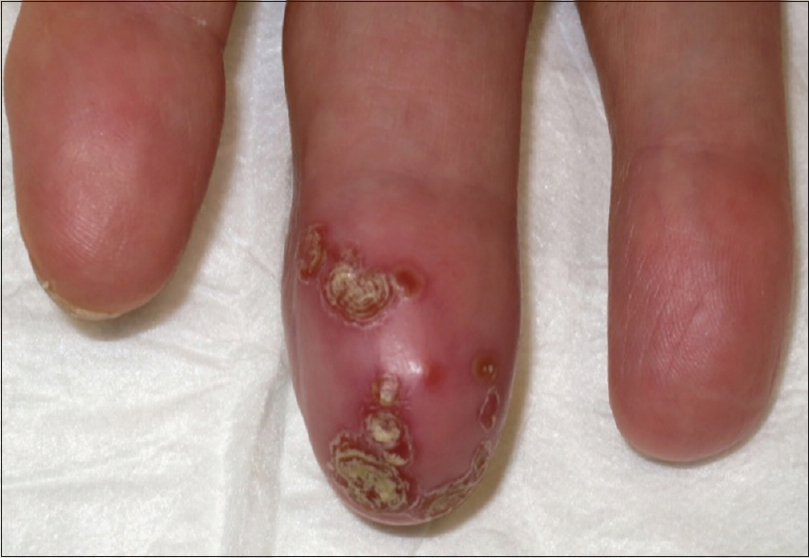 |
| Figure 1: Erythema and swelling of the right middle finger with annular crusts in a confluent pattern |
Two punch biopsies were performed. Histological examination revealed mild epidermal hyperplasia with a subcorneal pustule and a non-necrotizing granuloma in the dermo-hypodermic junction. Rest of the dermis showed a mild lymphoid infiltrate [Figure - 2]a and b]. The cultures in Sabouraud agar, Löwenstein–Jensen and blood agar media were non-yielding. The Tzanck test and the polymerase chain reaction for herpes simplex virus were also negative. In-house polymerase chain reaction amplification of mycobacterial 16S rRNA gene directly from solid tissue sample and subsequent gene sequencing using Sanger method in our Microbiology Unit confirmed the presence of Mycobacterium abscessus subsp. abscessus.
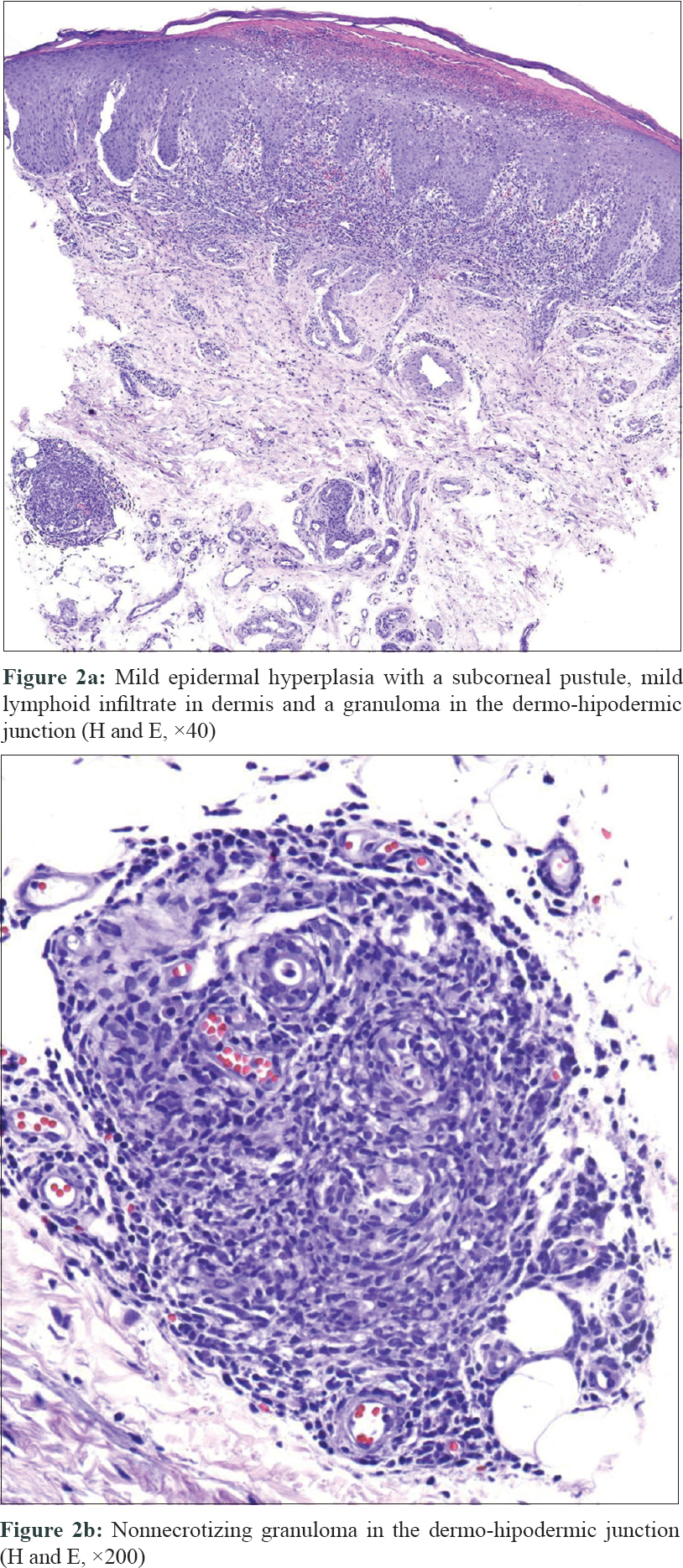 |
| Figure 2: |
Due to the lack of an antibiogram, we administered empiric treatment with clarithromycin 500 mg b.i.d. and ciprofloxacin 500 mg b.i.d. Amikacin was not initiated as the patient was reluctant to receive intravenous therapy. The finger showed rapid clinical improvement in the following weeks. However, only partial clinical response was observed after 4 months which prompted the addition of minocycline 100 mg b.i.d. to the current drugs. The patient completed a 6-month course of these three antibiotics with complete resolution of the lesions [Figure - 3].
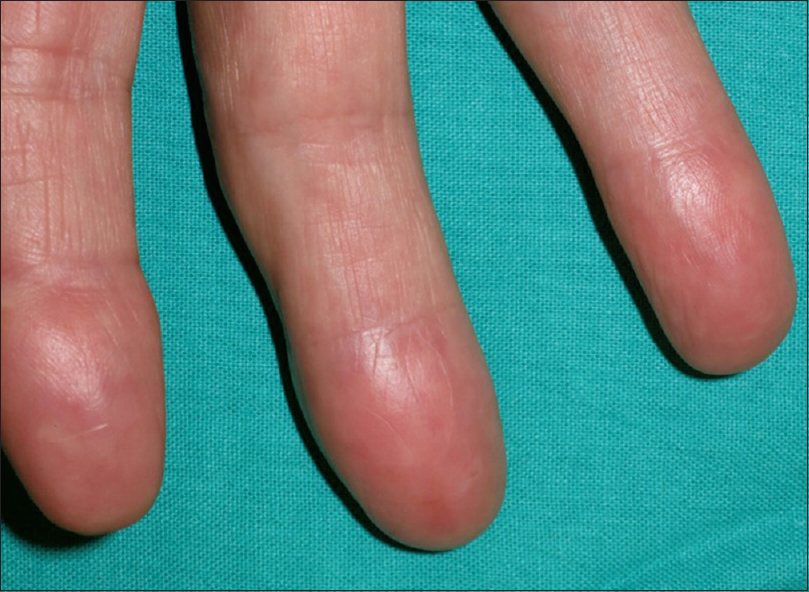 |
| Figure 3: Complete clinical resolution after 6 months with triple antibiotic therapy |
Mycobacterium abscessus is an atypical fast-growing mycobacteria, capable of producing progressive pulmonary disease and soft tissue infections in humans. This organism is ubiquitous in the environment and has been found in municipal and well water, soil and dust. An increased incidence of cutaneous nontuberculous mycobacterial infections has been documented recently.[1] In addition, several outbreaks of M.abscessus skin and soft tissue infections have also been recently reported, associated with medical procedures, cosmetic surgery, acupuncture and tattoos.[2],[3]
Cutaneous infectionby M. abscessus is characterized by nodules, recurrent abscesses and nonhealing ulcers. Multiple lesions may be observed.[4] The common differentials include bacterial and fungal infections, granulomatous processes and tumoral diseases, thus a high index of suspicion is necessary for diagnosis. The diagnosis may be confirmed by a positive culture of M.abscessus from drainage material, surgical debridement or skin biopsy. However, the isolation of M.abscessus is notoriously difficult and slow, so more rapid diagnostic methods, such as polymerase chain reaction, are being used. Differences in gene-sequence coding for 16S ribosomal RNA help differentiate among the different atypical mycobacteria subspecies.[4],[5]
In one of the largest series reported, histopathology revealed nodular, granulomatous or diffuse inflammation with mixed granulomas in 80% of the cases and abscesses with a mild granulomatous reaction in 15% of the biopsies. However, bacilli could be detected only in 27% of the specimens.[2]
M.abscessus is intrinsically resistant to several of the currently available antibiotics, including the classical antituberculous drugs,[4],[5] thus this condition is extremely difficult to treat. Intrinsic resistance is attributed to multiple factors like low permeability of the cell envelope, drug efflux pumps (ABC-type multidrug transporters), genetic polymorphism of antibiotic target genes and enzymes in the cytoplasm that neutralize antibiotics, such as aminoglycosides.[5] Also, inducible resistance for macrolides encoded by the erm gene has been reported and may explain the lack of efficacy of clarithromycin-based therapy in certain patients.
Successful treatment involves debridement, curettage of all infected tissue, excision of foreign bodies, as well as antimicrobial therapy for several months.[4] No controlled clinical trials of treatment for M. abscessus have been performed. If susceptibility testing shows sensitivity after prolonged incubation (14 days), this may predict a favorable outcome with a combination therapy of clarithromycin and amikacin, possibly supplemented with cefoxitin or a fluorquinolone.[5]
In conclusion, increased incidence of cutaneous nontuberculous mycobacterial infections has being reported in the past years. The diagnosis and treatment of skin and soft tissue infections caused by M. abscessus is a challenge for clinicians. Molecular biology techniques such as polymerase chain reaction offer high sensitivity and rapid results and represent an interesting alternative to the microbiological culture. A high suspicion index and awareness is necessary for diagnosis, especially in atypical clinical presentation, such as our case.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Wentworth AB, Drage LA, Wengenack NL, Wilson JW, Lohse CM. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: A population-based study. Mayo Clin Proc 2013;88:38-45.
[Google Scholar]
|
| 2. |
Rodríguez G, Ortegón M, Camargo D, Orozco LC. Iatrogenic Mycobacterium abscessus infection: Histopathology of 71 patients. Br J Dermatol 1997;137:214-8.
[Google Scholar]
|
| 3. |
Newman MI, Camberos AE, Ascherman J. Mycobacteria abscessus outbreak in US patients linked to offshore surgicenter. Ann PlastSurg 2005;55:107-10.
[Google Scholar]
|
| 4. |
Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: Comparison of clinical features, treatment, and susceptibility. Arch Dermatol 2006;142:1287-92.
[Google Scholar]
|
| 5. |
Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: A new antibiotic nightmare. J AntimicrobChemother 2012;67:810-8.
[Google Scholar]
|
Fulltext Views
2,393
PDF downloads
1,473





