Translate this page into:
Dowling-Degos disease and malignant melanoma: Association or mere coincidence?
2 Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Kanika Sahni
Department of Dermatology and Venereology, Room 4078A, Teaching Block, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029
India
| How to cite this article: Gupta V, Sahni K, Khute P, Sharma VK, Ali MF. Dowling-Degos disease and malignant melanoma: Association or mere coincidence?. Indian J Dermatol Venereol Leprol 2015;81:627-628 |
Sir,
Dowling-Degos disease is a rare genodermatosis characterized by reticulate hyperpigmentation of the flexures. Although it is a benign condition, a few cases of cutaneous malignancies including squamous cell carcinoma and keratoacanthoma have been reported in these patients.[1],[2],[3] We report a case of metastatic amelanotic melanoma in a patient with Dowling-Degos disease. We were unable to find any previously published reports of such an association.
A 51-year-old man presented to us for the evaluation of nodular lesions on his scrotum. The lesions first appeared 1½ years ago as small skin-coloured to erythematous papules which rapidly enlarged to form nodules that later ulcerated. He had no systemic ailments. On examination, there were multiple, erythematous, firm to hard nodules and plaques on the left side of the scrotum. Many of these were ulcerated. There were smaller papules on the periphery with a few scattered nodules in the adjacent inguinal region [Figure - 1]a. The bilateral inguinal lymph nodes were enlarged (1.5 cm – 2 cm), hard, mobile and non-tender. Incidentally, he was found to have multiple pitted scars over the face, back, buttocks, pubic region and medial thighs [Figure - 1]b. The forehead, axillae, thighs and back also showed follicular keratotic papules and pin-point hyperpigmented macules [Figue 1c. These lesions were present since adolescence and there was a history of similar lesions in his younger brother. A biopsy taken from the scrotal nodule showed nodular aggregates of tumor cells arranged in nests. These cells had pleomorphic vesicular nuclei, prominent eosinophilic nucleoli and a moderate amount of cytoplasm with focal melanin deposits [Figure - 2]a. Immunohistochemistry was positive for HMB-45 and S-100, and negative for cytokeratin 7, 20 and prostate specific antigen [Figure - 2]b. A fine needle aspiration cytology from the left inguinal lymph node showed similar atypical cells. A biopsy from a keratotic papule and hyperpigmented macule showed elongation and branching of rete pegs along with basal hyperpigmentation [Figure - 3]. A whole-body positron emission tomography scan showed hypermetabolic cutaneous-subcutaneous thickening in the inguino-scrotal region on both sides, along with increased uptake in the pelvic and inguinal lymph nodes, liver and right scapula. On the basis of clinical examination and investigations, a diagnosis of Dowling-Degos disease and primary cutaneous amelanotic malignant melanoma with nodal and visceral metastases was made. The patient was planned for 3-weekly cycles of dacarbazine.
 |
| Figure 1: (a) Multiple grouped shiny skin-colored to erythematous nodules and plaques on the left side of the scrotum with adjacent papules and nodules. (b) Face showing discrete, hyperpigmented macules on the forehead. Pitted scars prominent on the nose, perinasal and perioral regions. (c) Brown hyperpigmented macules in the right axilla |
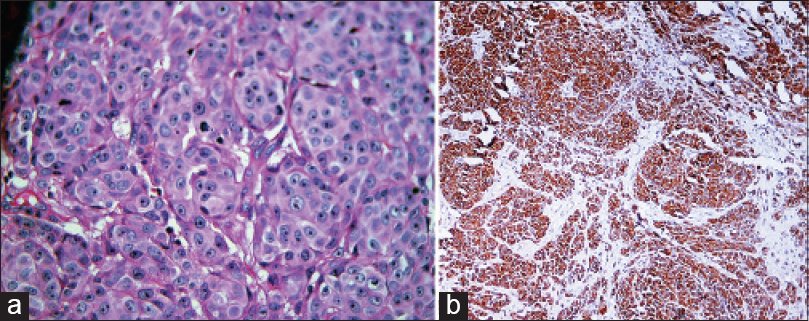 |
| Figure 2: (a) Nests of tumor cells having pleomorphic vesicular nuclei, prominent eosinophilic nucleoli and moderate amount of cytoplasm (H and E stain, ×400), (b) HMB-45 monoclonal antibody staining the cytoplasm of the tumor cells (×100) |
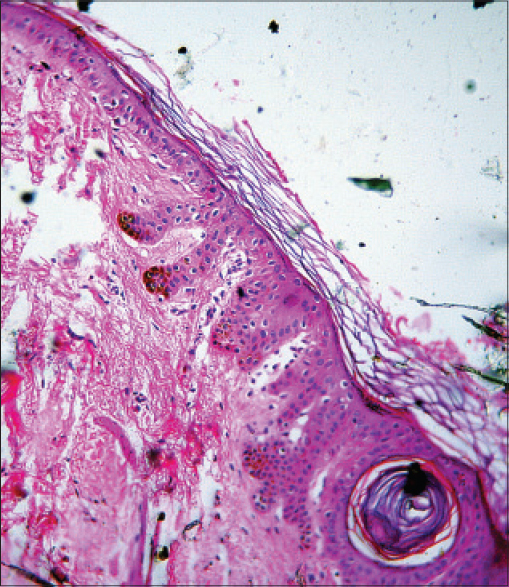 |
| Figure 3: Elongation and branching of rete pegs with basal hyperpigmentation, consistent with Dowling-Degos disease |
Dowling-Degos disease is a rare, autosomal dominant genodermatosis characterized by reticulate hyperpigmentation of flexural sites such as the neck, axilla, cubital fossa and groin.[4] A loss-of-function mutation in keratin-5 (KRT5) gene is the most well characterized genetic defect.[5],[6] Although the pigmentation observed in our patient was not in a classic reticulate pattern, the histological features were suggestive of Dowling-Degos disease. This pattern of pigmentation has been described as 'dappled' pigmentation.[3] The melanoma lesions were unusual in being amelanotic. However, the histologic findings were diagnostic of melanoma which was further confirmed by positive immunohistochemistry for HMB-45 and S-100.
There are only a few case reports of cutaneous malignancies occurring in association with Dowling-Degos disease.[1],[2],[3] Fenske et al. described a case of multiple keratoacanthomas on the legs and back of a patient with Dowling-Degos disease associated with hidradenitis suppurativa.[1] They speculated a single underlying defect in follicular epithelial proliferation and keratinization as the basis for the coexistence of these disorders. Hohmann et al. reported a case of solitary keratoacanthoma on the foot associated with this disease in a 53-year-old man.[2] Two adenoid squamous cell carcinomas have been reported to arise from pigmented lesions on the buttock in a 64-year-old Japanese man. The authors hypothesized that squamous cell carcinomas developed in relation to a nevoid anomaly of the pilosebaceous unit in Dowling-Degos disease.[3]
We speculate that the melanoma may have originated from the follicular unit in our patient too, more specifically from the amelanotic melanocytes (melanocyte stem cells) of the outer root sheath of the follicle. Of note, the KRT5 gene responsible for Dowling-Degos disease has been found to have reduced expression in metastatic melanomas.[7] This could be an explanation for the association of malignant melanoma with Dowling-Degos disease in our patient. However, we could not perform a KRT5 gene analysis in our patient due to lack of facilities. To summarize, the underlying pathomechanism behind this association is not entirely clear, but a follicular pathology and defective keratinisation with KRT5 gene mutation could be speculated as the common link between Dowling-Degos disease and malignant melanoma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Fenske NA, Groover CE, Lober CW, Espinoza CG. Dowling-Degos disease, hidradenitis suppurativa, and multiple keratoacanthomas. A disorder that may be caused by a single underlying defect in pilosebaceous epithelial proliferation. J Am Acad Dermatol 1991;24:888-92.
[Google Scholar]
|
| 2. |
Hohmann CB, Köche B, Bonamigo RR, Dornelles ST, Cattani CA. Case for diagnosis. Dowling-Degos disease. An Bras Dermatol 2010;85:241-3.
[Google Scholar]
|
| 3. |
Ujihara M, Kamakura T, Ikeda M, Kodama H. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol 2002;147:568-71.
[Google Scholar]
|
| 4. |
Bhagwat PV, Tophakhane RS, Shashikumar BM, Noronha TM, Naidu V. Three cases of Dowling Degos disease in two families. Indian J Dermatol Venereol Leprol 2009;75:398-400.
[Google Scholar]
|
| 5. |
Betz RC, Planko L, Eigelshoven S, Hanneken S, Pasternack SM, Bussow H, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet 2006;78:510-9.
[Google Scholar]
|
| 6. |
Verma S, Pasternack SM, Rütten A, Ruzicka T, Betz RC, Hanneken S. The first report of KRT5 mutation underlying acantholytic Dowling-Degos disease with mottled hypopigmentation in an Indian family. Indian J Dermatol 2014;59:476-80.
[Google Scholar]
|
| 7. |
Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics 2008;1:13.
[Google Scholar]
|
Fulltext Views
2,937
PDF downloads
1,177





