Translate this page into:
Drug reaction with eosinophilia and systemic symptoms due to lercanidipine
Correspondence Address:
Raoudha Slim
Department of Clinical Pharmacology, Faculty of Medicine of Sousse, Avenue Mohamed Karoui, BP 126, Sousse 4002
Tunisia
| How to cite this article: Slim R, Fathallah N, Larif S, Ben Salem C. Drug reaction with eosinophilia and systemic symptoms due to lercanidipine. Indian J Dermatol Venereol Leprol 2016;82:324-326 |
Sir,
Dihydropyridine calcium channel blockers are among the drugs of choice for the treatment of hypertension because of their efficacy and potential beneficial effects. Lercanidipine is a third-generation dihydropyridine calcium channel blocker and is usually well-tolerated. However, dihydropyridine calcium channel blockers can induce adverse skin reactions. A maculopapular rash is the most common manifestation reported.[1] Rarely, systemic reactions have been described. Herein, we report a patient who developed a drug reaction with eosinophilia and systemic symptoms (DRESS) after intake of lercanidipine.
An 81-year-old man with a 12-month-history of hypertension was being treated with delapril and indapamide. Because of poor blood pressure control, he was switched to a regimen of lercanidipine 10 mg once daily. There was no history of drug allergy. The patient denied the use of any other medications. Three weeks later, he developed a febrile skin eruption. Physical examination showed generalized maculopapular exanthema, facial edema with desquamation [Figure - 1], cervical lymphadenopathy and oral mucosal involvement. Laboratory investigations revealed leukocytosis (17.6 × 109/L) with eosinophilia (5.1 × 109/L). Creatinine level was 212 μmol/L (normal range: 70–120 μmol/L) and urea nitrogen was 24.17 mmol/L (normal range: 2.5–7.5 mmol/L). Liver enzymes were within normal limits, except for γ-glutamyl transferase 186 IU/L (normal range: 10–50 IU/L) and alkaline phosphatase 463 IU/L (normal range: 98–279 IU/L). Viral serology including hepatitis B and C, cytomegalovirus, Epstein–Barr virus, parvovirus B19 and human herpes virus 6 were negative. Tests for antinuclear antibody and blood culture were also negative. A skin biopsy showed hyperkeratosis and spongiosis along with edema in the epidermis and lymphocytic infiltration with numerous eosinophils in the upper dermis and around the subepidermal vessels [Figure - 2]. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was suspected; lercanidipine was stopped and treatment with prednisolone, 20 mg/day orally was started. The patient's condition improved in 4–5 days, the rash cleared, erythema decreased and oral ulceration subsided. He was discharged on a tapering course of prednisolone. After 3 weeks, his laboratory test results returned to normal and his hypertension was stabilized with captopril. A patch test with lercanidipine was requested but the patient refused additional investigations.
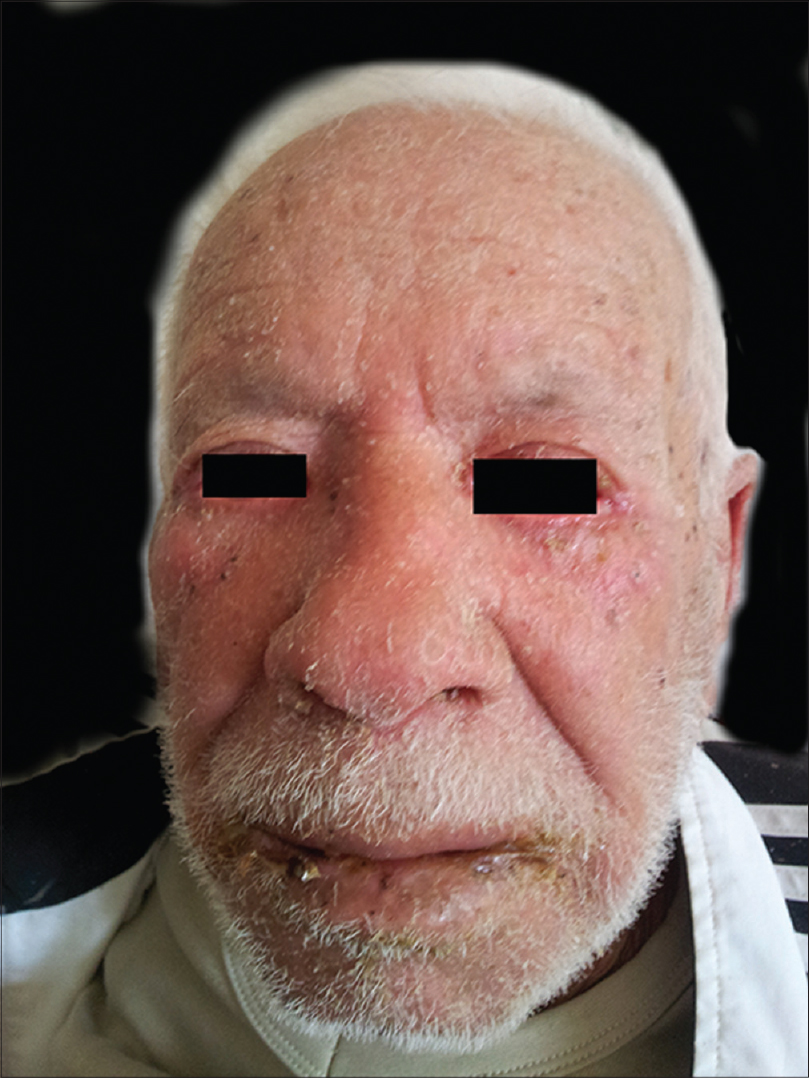 |
| Figure 1: Marked facial edema with desquamation |
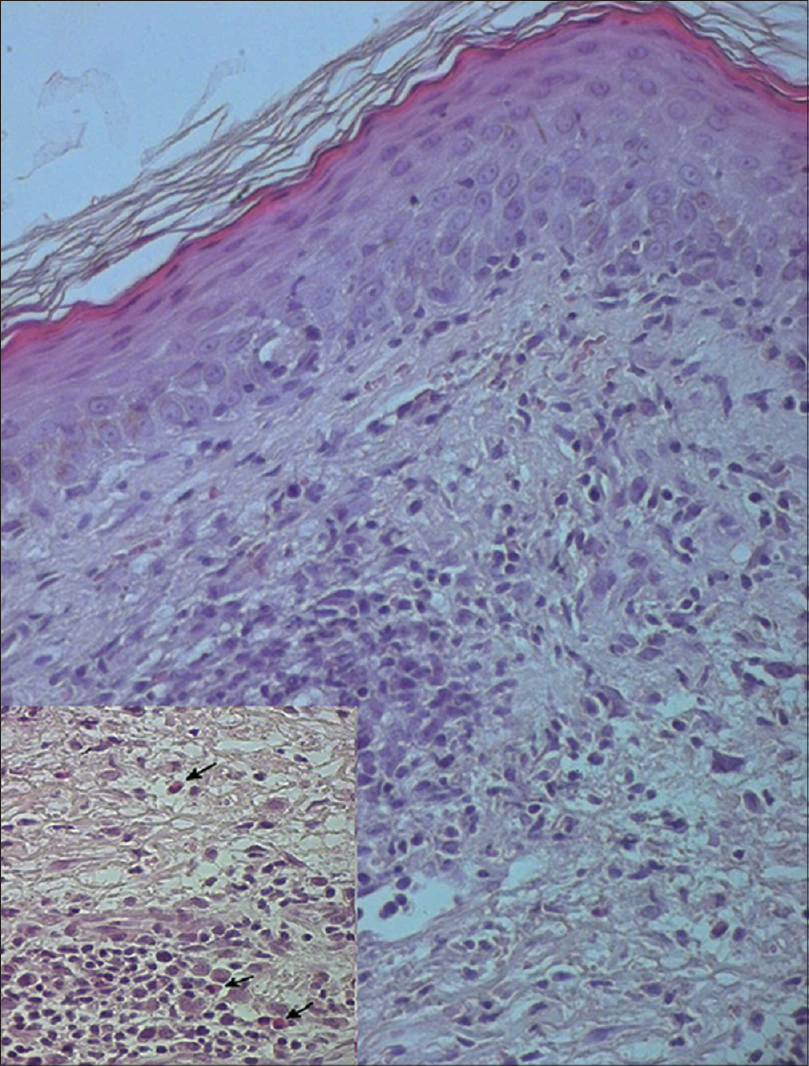 |
| Figure 2: Hyperkeratosis, spongiosis and edema in the dermis (H and E, ×100). Inset (lower left); shows lymphocytic infiltration with numerous eosinophils (arrow) in the upper dermis (H and E, ×400) |
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome or drug-induced hypersensitivity syndrome (DHS) is a severe and potentially life-threatening disease presenting with skin rash, eosinophilia and systemic involvement occurring 1–8 weeks after drug introduction. The most frequently implicated drugs are anticonvulsants, sulfonamides, antivirals and allopurinol. The pathophysiological pathways are not fully understood and may be multifactorial involving immunological mechanisms, particularly drug detoxification pathways and sequential reactivation of herpes viruses.[2]
Lercanidipine is a dihydropyridine calcium channel blocker indicated for the treatment of mild-to-moderate hypertension. The drug is well tolerated causing much less peripheral edema than amlodipine in most trials. Other lercanidipine-associated adverse events include peripheral flushing, palpitation and dizziness. Cutaneous eruptions related to calcium channel blockers are rare. Maculopapular rash is the most common dermatologic manifestation; however, serious adverse events such as anaphylaxis, Stevens–Johnson syndrome and toxic epidermal necrolysis have occasionally been reported.[1]
Only a few previous cases of drug reaction with eosinophilia and systemic symptoms syndrome have been reported following exposure to a calcium channel blocker. Diltiazem was the most frequently incriminated calcium channel blocker agent.[3] The onset of the eruption occurred 2–10 days after starting diltiazem. Liver involvement occurred in the majority of the cases. Recently, Moriya et al. presented a case of drug reaction with eosinophilia and systemic symptoms syndrome, which occured 19 months after initiating azelnidipine and verapamil hydrochloride, which appears to be a very long delay in the onset of the syndrome.[4] Our patient's symptoms started 3 weeks after lercanidipine initiation. It was classified as a definitive case of drug reaction with eosinophilia and systemic symptoms according to the RegiSCAR scoring system.[5] According to the Naranjo probability scale, the adverse drug reaction was considered probable.[6]
In conclusion, lercanidipine can be added to the list of drugs known to induce drug reaction with eosinophilia and systemic symptoms syndrome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Tuchinda P, Kulthanan K, Khankham S, Jongjarearnprasert K, Dhana N. Cutaneous adverse reactions to calcium channel blockers. Asian Pac J Allergy Immunol 2014;32:246-50.
[Google Scholar]
|
| 2. |
Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol 2013;68:693.e1-14.
[Google Scholar]
|
| 3. |
Knowles S, Gupta AK, Shear NH. The spectrum of cutaneous reactions associated with diltiazem: Three cases and a review of the literature. J Am Acad Dermatol 1998;38(2 Pt 1):201-6.
[Google Scholar]
|
| 4. |
Moriya M, Aihara M, Imai M, Ikezawa Y, Matsukura S, Kanbara T, et al. Atypical drug-induced hypersensitivity syndrome with human herpesvirus 6 reactivation due to calcium blockers. J Dermatol 2013;40:129-30.
[Google Scholar]
|
| 5. |
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br J Dermatol 2007;156:609-11.
[Google Scholar]
|
| 6. |
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. Amethod for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
[Google Scholar]
|
Fulltext Views
4,244
PDF downloads
1,942





