Translate this page into:
Drug reaction with eosinophilia and systemic symptoms with toxic epidermal necrolysis like rash in systemic sclerosis and overlapping sjogren’s syndrome: A double trouble triggered by a herbal medicine
Corresponding author: Dr. Logamoorthy R, Department of Dermatology Venereology and Leprosy, Sri Manakula Vinayagar Medical college & Hospital, Madagadipet, Kalitheerthalkuppam, Pondicherry, India. logamoorthy.r@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Youvalakshmi S, Logamoorthy R, Karthikeyan K. Drug reaction with eosinophilia and systemic symptoms with toxic epidermal necrolysis like rash in systemic sclerosis and overlapping sjogren’s syndrome: A double trouble triggered by a herbal medicine. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_726_2024
Dear Editor,
Toxic epidermal necrolysis (TEN) is a severe cutaneous adverse reaction characterised by extensive full-thickness skin and mucous membrane necrosis, resulting in widespread exfoliation and possible sepsis. TEN-like presentations, associated with auto-immune diseases, are more commonly reported with acute lupus erythematosus and less likely with systemic sclerosis.1 TEN-like manifestations in connective tissue disorders and drug-induced TEN are clinically indistinguishable. Hence, a high index of suspicion is important as the prognosis of the disease will be altered by early diagnosis and prompt management. In this case report, we present a case of drug-induced TEN in a patient with systemic sclerosis.
A 33-year-old female, a known case of systemic sclerosis since the past two years, presented with reddish raised lesions all over the body with facial oedema and oral erosions for 20 days following the intake of a native medication (Smilax china). She was on irregular treatment with methotrexate and nicardipine for systemic sclerosis for the past one year. On cutaneous examination, the patient had a diffuse maculopapular rash over the anterior and posterior trunk, upper limb, neck and bilateral thighs, suggesting a drug-induced exanthem [Figures 1a and 1b]. The clinical and laboratory findings are tabulated in Table 1. The complete hemogram revealed anaemia with a haemoglobin level of 9.8 g/dL (12–15 g/dL), a white blood cell count of 6200 cells/cu.mm (4000–11000 cells/cu.mm) with 8% (0–6%) eosinophilia, a platelet count of 373,000 cells/cu.mm (1,50,000–4,50,000 cells/cu.mm). The liver function test showed elevated Aspartate aminotransferase (AST) at 50 IU/L (Normal: 0–35 IU/L) and Alanine aminotransferase (ALT) at 331 IU/L (Normal: 0–35 IU/L). A diagnosis of probable DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms) was made due to the presence of eosinophilia and hepatitis, with a Regiscar score of 4. The patient was admitted and started on oral prednisolone (0.5 mg/kg) and IV ceftriaxone 1 g once daily for heavy Escherichia.coli growth in vaginal swabs taken from erosions over the vaginal mucosa. After two days, the lesions progressed to vesiculobullous eruptions with epidermal detachment all over the body. On examination, multiple vesicles and bulla were present all over the body, with sheets of necrosis and spots seen over the trunk and extremities [Figures 2a and 2b]. The biopsy from the posterior trunk showed subepidermal blister formation containing lymphocytes, macrophages and occasional acantholytic cells along with RBCs and eosinophilic fluid. The superficial dermis shows minimal dermal oedema with mild perivascular and periadnexal inflammatory infiltrate composed of lymphocytes and plasma cells within a thick, dense collagenised stroma suggestive of systemic sclerosis with toxic epidermal necrolysis [Figure 3]. The Naranjo score was 5, indicating a probable causal relationship between the drug and toxic epidermal necrolysis.2 Ceftriaxone was stopped in view of worsening of pre-existing drug rash, following which the lesions stopped progressing after 48 hours. High resolution computed tomography (HRCT) showed bilateral basal sub-pleural honeycombing, which was indicative of interstitial lung disease. The patient was treated with dexamethasone 8 mg once daily for 6 days and the patient was later switched to oral prednisolone 40 mg once daily at the time of discharge, with mycophenolate mofetil 500 mg twice daily, nintedanib 100 mg twice daily and doxophylline 200 mg twice daily.
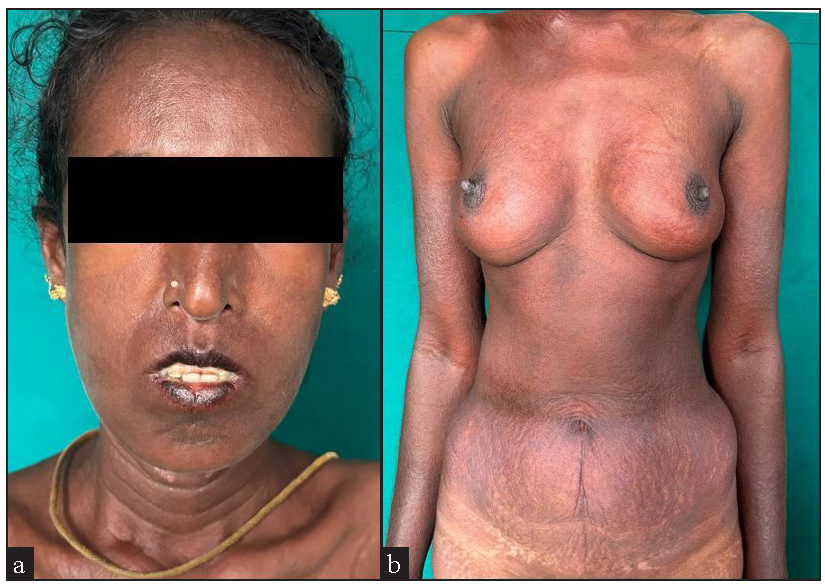
- Woman with systemic sclerosis with diffuse maculopapular rash following intake of herbal medicine (Smilax china) (a) over the face with chapped lips and (b) over the trunk.
| Clinical Manifestations and Investigations | |
|---|---|
| Face | Elongated face, pinched nose, pursed lips and flattening of the nasolabial fold |
| Cutaneous examination | Skin thickening and tightening of the face and extremities, salt-and-pepper pigmentation over the flexural aspect and nape of the neck and right forearm. |
| Oral cavity | Restriction of mouth opening and perioral furrowing |
| Hand | Pitted scars over the fingertips and sclerodactyly |
| Positive signs | Ingram’s sign and Barnet’s neck sign |
| Modified Rodnan Skin Score (MRSS) | 17/51 |
| Nailfold capillaroscopy | Drop outs present with few haemorrhages |
| EULAR criteria (2013) | 19 |
| ANA (Indirect Immunofluorescence) |
4+ homogenous pattern (1:80 dilution) Positive for anti-Scl-70, anti-RO & anti-SSA antibodies |
EULAR: European Alliance of Associations for Rheumatology, RO: ribonucleoprotein, SSA: Sjogren’s syndrome antibody.
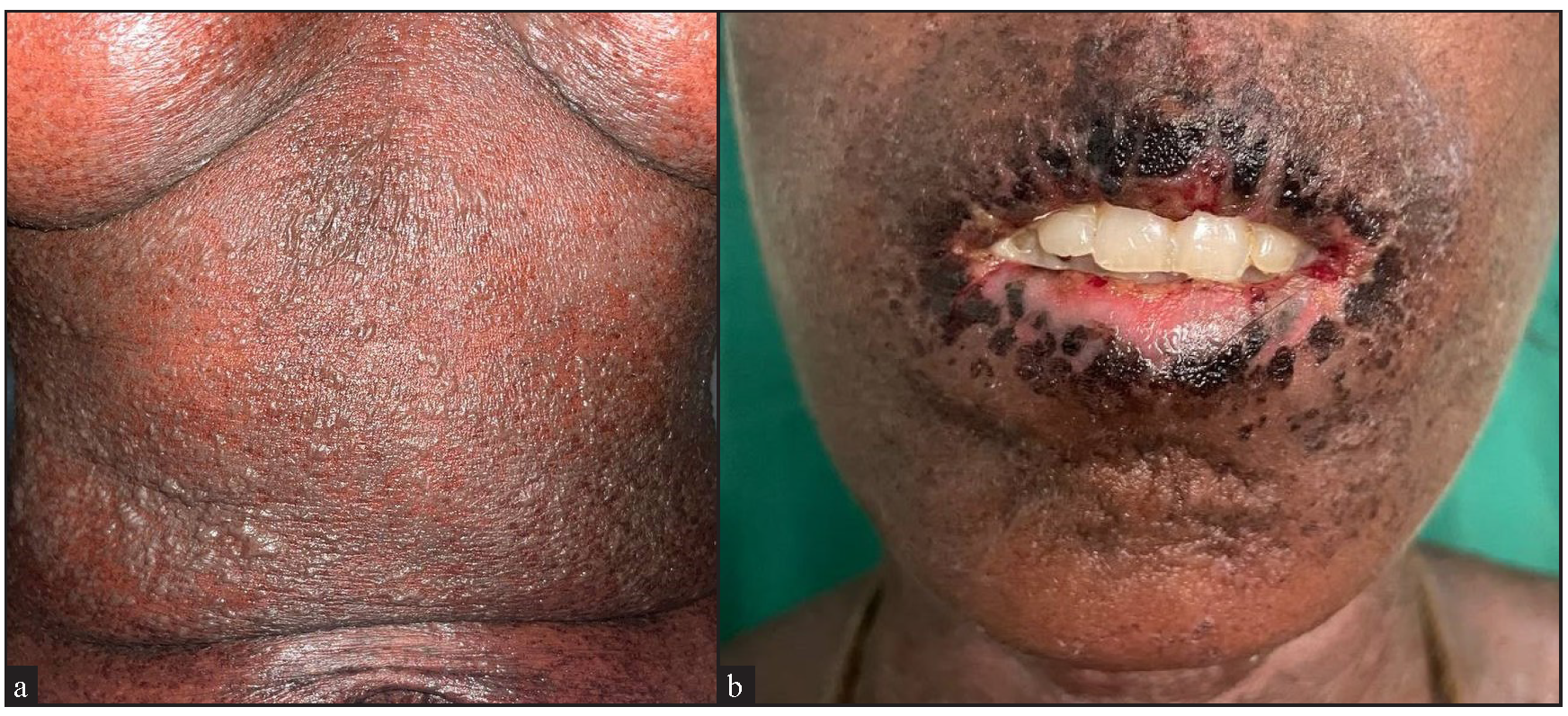
- (a) Confluent vesicles and bullae over the trunk with sheets of necrosis on a background of erythema and (b) oral erosions with haemorrhagic and dried up crust over the lips and perioral area suggestive of toxic epidermal necrolysis.
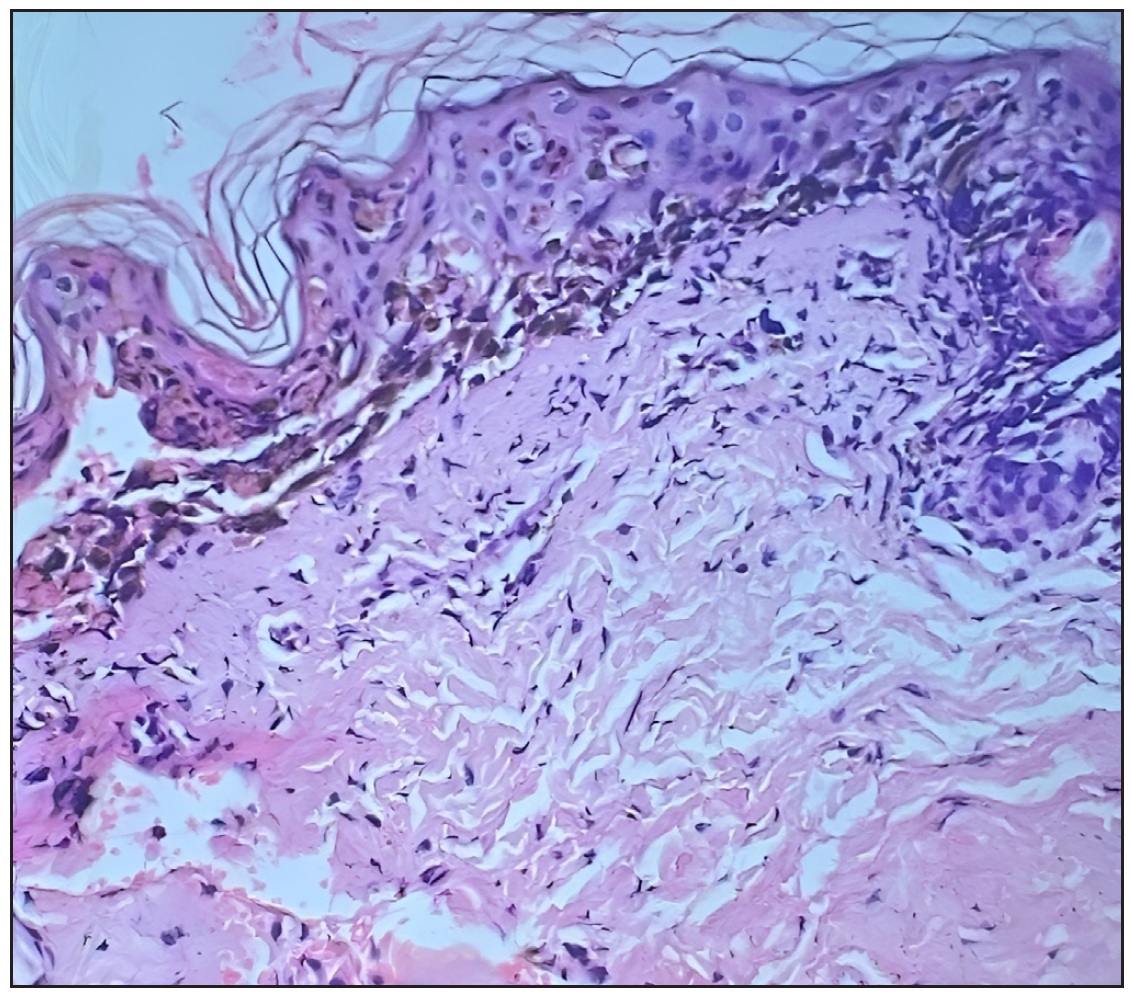
- Subepidermal blister with basket weave orthokeratosis, apoptotic keratinocytes with dense lymphocytic infiltrate in the superficial dermis, dermal oedema, and dense collagenised stroma (Haematoxylin and eosin, 400x)
Systemic sclerosis is an autoimmune disease that causes fibrosis of the skin and internal organs with associated microvascular damage. Bullous lesions in systemic sclerosis are a rare manifestation. It can occur due to several pathologic mechanisms like drug-induced, autoimmune, secondary to fibrosis and lymphatic obstruction.3,4 TEN is a dreaded condition resulting in widespread blistering eruptions. TEN-like lesions are more likely to occur in patients with connective tissue disease as in TEN-like acute and subacute cutaneous lupus erythematosus. Aiempanakit et al. described a patient with systemic sclerosis developing TEN-like cutaneous lupus erythematosus.5 In our case, the patient was diagnosed with drug-induced TEN with systemic sclerosis and secondary Sjogren syndrome. The difference between TEN associated with connective tissue disease and drug-induced TEN is shown in Table 2. However, these differences are subjective and theoretical.6,7 The primary therapy for drug-induced TEN is the withdrawal of the culprit drug followed by barrier nursing, wound care, fluid and electrolytes correction, analgesic support and intensive care for systemic complications. Other treatment options include systemic corticosteroids, cyclosporine, IV immunoglobulins and plasma exchange. Further, the active ingredient of the native medication is the herb Smilax china. We have performed a literature search for drug rash due to smilax china but were unable to find any TEN or DRESS reported previously to smilax china. It is a popular herb used in Siddha, Ayurveda and Chinese medicine. It is to be remembered that such serious adverse reactions can occur with herbal medications as well.
| TEN | TEN associated with CTD | |
|---|---|---|
| Pathophysiology |
Genetical predisposition in special haplotypes, HLAB *1502 and HLA B*5801 Activation of soluble Fas ligand pathway Increased granulysin and perforin |
Depends on the underlying connective tissue disorder: Lupus Erythematosus: ultraviolet rays-induced apoptosis and necrosis; increases CCL27 (activation of autoimmune T cells) Systemic Sclerosis: bullous lesions are a rare entity |
| History | History of high-risk drug intake | No drug history |
| Sex predominance | Female>male (1.5:1) | Female>male (9:1) |
| Mucosal involvement | Severe | Mild while compared to drug-induced TEN |
| Laboratory | Negative for LE-related antibodies | ANA +, ds DNA +, C3 and C4 |
| Histopathology | Interface dermatitis, along with vacuolar basal cell degeneration and scattered necrotic keratinocytes in the epidermis | Epidermal necrosis involving the entire epidermis, vacuolar degeneration of basal keratinocytes |
| Direct IF | Negative | Dense granular IgM, IgA and C3 deposits along the dermo-epidermal junction. |
| Management | Stop culprit drug, shorter course of steroids, cyclosporine, IVIG and plasmapheresis | Steroids (long duration) and immunosuppressants |
CTD: connective tissue disease, HLA B: Human Leukocyte Antigen B, ANA: antinuclear antibodies, ds DNA: double-stranded deoxyribonucleic acid, IF: immunofluorescence, IVIG: intravenous immunoglobulin.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus. 2010;19:1050-70.
- [CrossRef] [PubMed] [Google Scholar]
- Causality assessment of cutaneous adverse drug reactions. Ann Dermatol. 2011;23:432-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diffuse systemic sclerosis with bullous lesions without systemic manifestations. An Bras Dermatol. 2013;88:78-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus in a patient with progressive systemic sclerosis. Lupus. 2018;27:1860-3.
- [CrossRef] [PubMed] [Google Scholar]
- Association of antinuclear antibodies with toxic epidermal necrolysis – A novel manifestation. East J Med Sci. 2020;5:61-5.
- [Google Scholar]
- Toxic epidermal necrolysis like acute cutaneous lupus erythematous or drug-induced toxic epidermal necrolysis: a case report of a diagnostic dilemma. Int J Res Dermatol. 2020;6:563-6.
- [Google Scholar]





