Translate this page into:
Effect of low-dose acitretin treatment on pituitary hormones in psoriasis vulgaris: A retrospective study
2 Department of Dermatology, Duzce University, School of Medicine, Duzce, Turkey
3 Department of Internal Medicine, Istanbul Medeniyet University, School of Medicine, Istanbul, Turkey
4 Department of Dermatology, Van Yuzuncu Yil University, Van, Turkey
5 Department of Biochemistry, Van Yuzuncu Yil University, Van, Turkey
6 Department of Endocrinology, Kecioren Research and Training Hospital, Ankara, Turkey
Correspondence Address:
Ayse Serap Karadag
Department of Dermatology, Medeniyet University Faculty of Medicine, Istanbul 34730
Turkey
| How to cite this article: Karadag AS, Ozlu E, Kostek O, Bilgili SG, Balaharoglu R, Ertugrul DT. Effect of low-dose acitretin treatment on pituitary hormones in psoriasis vulgaris: A retrospective study. Indian J Dermatol Venereol Leprol 2019;85:300-304 |
Abstract
Background: It has been reported that retinoids may lead to hormonal alterations.
Aim: In this retrospective study, we aimed to study the effect of acitretin on pituitary hormones in psoriasis patients.
Methods: Out of 50 patients intended to be studied, blood samples of 43 patients could be tested before and after 3 months of acitretin therapy (0.2 to 0.5 mg/kg/day).
Results: Patients mean ± standard deviation ages and female/male ratio were 46 ± 17 years and 19/24, respectively. After treatment with acitretin, gamma-glutamyltransferase, alkaline phosphatase, total cholesterol and triglyceride levels increased significantly (P < 0.05). After treatment, total protein, free thyroxine (T4) levels decreased significantly (P < 0.05). No significant differences were observed between before–after acitretin treatment regarding pituitary hormone levels in psoriasis patients (P > 0.05).
Limitations: The retrospective nature of the study, inability to retest blood samples of 7 patients at 3 months post treatment, low dose and short duration of acitretin treatment were limitations of this study.
Conclusion: This study showed that pituitary hormones were not affected except free T4 (thyroid hormone) by acitretin treatment. Further experimental and clinical studies are needed to clarify the effect of acitretin on pituitary hormones.
Introductıon
The relationship of psoriasis with the endocrine system has attracted the interest of many researchers in recent years.[1] There is some evidence suggesting that psoriasis becomes worse during puberty, pregnancy and the menopausal period.[2],[3] The role of stress hormones has also been investigated in the pathogenesis of psoriasis.[4],[5] Altered thyroid hormone levels during the active phase of psoriasis and regression of psoriasis with anti-thyroid therapy indicate a relationship between thyroid hormones and severity of psoriasis.[6],[7] Evers et al. reported decreased cortisol response to stress in psoriasis.[8] Another study found a relationship between psoriasis and hypothalamic–pituitary–adrenal axis, and that higher bedtime cortisol levels were associated with increased severity of psoriasis.[9] Previous studies have shown that drugs used in the treatment of psoriasis may alter several hormone levels, including pituitary hormones.[10],[11]
Retinoic acid receptors and RXR are expressed in the pituitary gland in the embryo, as well as in adults.[12] Vitamin A and its derivatives have also been shown to have regulatory effects on the endocrine system in rats.[13],[14] Similarly, retinoic acid has been demonstrated to modulate some pituitary hormone receptor levels in rat granulosa cells.[15] Retinoic acid levels were found to be higher in psoriatic plaques compared with normal skin.[16]
Although there are many studies showing that hormonal changes play a key role in the pathogenesis of psoriasis, the number of studies investigating the effects of acitretin therapy on pituitary hormone levels in patients with psoriasis is limited. In this study, we investigated the effects of acitretin therapy on pituitary hormone levels in patients with psoriasis.
Methods
Study design and patients
This was a retrospective noncontrolled study conducted on psoriasis patients presenting to the dermatology department of Yuzuncuyıl University, Van, Turkey. The study protocol was in accordance with the Declaration of Helsinki and was approved by local ethics committee. Out of 50 patients intended to be studied, blood samples before starting, and 3 months after treatment, could be drawn only for 43 patients. Inclusion criteria for enrollment into the study included moderate-to-severe plaque-type psoriasis of more than 6 months duration. Exclusion criteria were as follows: age younger than 18 years, the presence of pregnancy or lactation, infections, a history of previous treatment history within the last 12 weeks before enrollment into the study including phototherapy, immunosuppressive and/or immunomodulating drugs and biological agents. Subjects with chronic diseases such as endocrine, cardiovascular, hepatic, hematologic, renal, thyroid disease and cancer were also excluded from the study.
Acitretin therapy and clinical assessments
Acitretin at a dose of 0.2–0.5 mg/kg/day was orally administered to patients with psoriasis vulgaris. The hormonal and biochemical measurements were performed at baseline and after 3 months of acitretin treatment. The clinical improvement was measured by Psoriasis Area and Severity Index.
Biochemical and hormonal measurements
Biochemical and hormonal parameters were: free T3 (fT3), free T4 (fT4), thyroid-stimulating hormone, thyroglobulin, anti-thyroid peroxidase and anti-thyroglobulin, total testosterone, estradiol, luteinizing hormone, follicle-stimulating hormone, sex hormone-binding globulin, cortisol, adrenocorticotropic hormone, hemoglobin, creatinine, alanine aminotransferase and aspartate aminotransferase, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol. Fasting blood samples were obtained by venipuncture of the large antecubital veins, without stasis and after 12 hours fasting. Fasting serum glucose, total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, alanine aminotransferase and aspartate aminotransferase concentrations were measured spectrophotometrically using an automatic analyzer, and serum glucose levels were measured by the hexokinase method (Architect ci16200 integrated system; Abbott Laboratories, Medical Diagnostics Products, New Jersey, USA). Thyroid-stimulating hormone (normal 0.3–3.6 mIU/l), fT3 (normal 2.2–4.2 pg/ml), fT4 (normal 0.65–1.7 ng/dl), anti-thyroid peroxidase (normal 1–500 IU/ml) and anti-thyroglobulin (normal 5–100 IU/ml), levels of estradiol (reference range 27–433 pg/ml), follicle-stimulating hormone (reference range 1.79–22.51 mIU/ml), luteinizing hormone (normal 0.2–250 mIU/ml), prolactin (normal 1.2–58.64 ng/ml), total testosterone (normal 0.1–0.75 ng/ml), sex hormone-binding globulin levels (normal 16–110 μmol/l) and cortisol (normal 6.7–22.6 μg/dl) were measured using chemiluminescence microparticle immunoassay method (Architect ci16200 integrated system; Abbott Laboratories, Medical Diagnostics Products, New Jersey, USA). Adrenocorticotropic hormone (normal 4.5–48.8 pg/ml) and thyroglobulin (normal 0.2–70 ng/ml) were measured using chemiluminescence enzyme immunoassay (Immulite 2000, Siemens Diagnostics, USA).
Limitations
The retrospective nature of the study, inability to retest blood samples of 7 patients 3 months post-treatment, low dose and short duration of acitretin treatment were limitations of this study.
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences version 15.0 software (SPSS Inc., Chicago, IL, USA). The normality of data was analyzed using the Kolmogorov–Smirnov test. All numerical variables following a normal distribution were expressed as the mean ± standard deviation, while data that were not normally distributed were expressed as the median (interquartile range). The paired sample t-test was used to compare pretreatment and posttreatment values for hormonal and biochemical data with homogenic variability. The Wilcoxon signed-rank test was used to analyze data with skew distribution.
Results
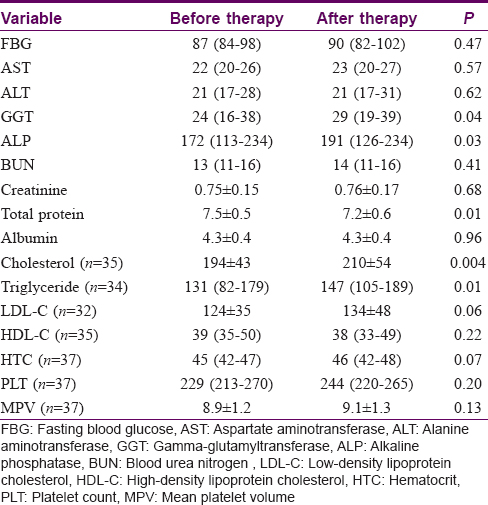
Our study included 43 moderate-to-severe plaque-type psoriasis patients (24 males, 19 females). The mean ± SD ages of psoriasis patients were 46 ± 17 years. After 3 months acitretin treatment, the median (range) Psoriasis Area and Severity Index significantly decreased from 15 ± 3.2 (8–20) to 5 ± 2.4 (2.1–9.4) [Table - 1].

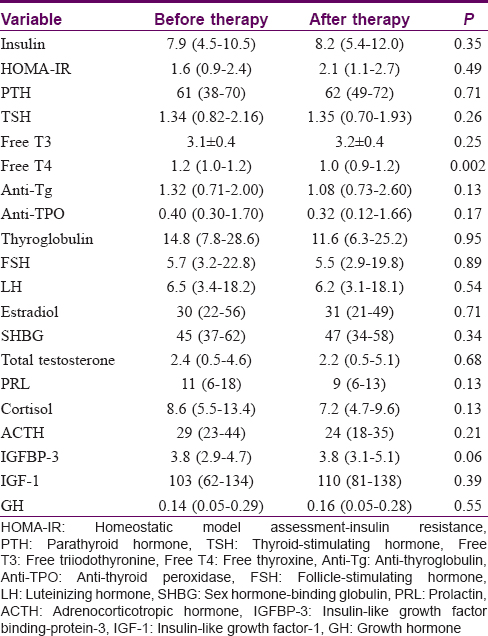
The gamma-glutamyltransferase, alkaline phosphatase, total cholesterol and triglyceride levels were significantly lower in the pretreatment serum of the patients when compared to their posttreatment levels (P < 0.05) [Table - 2]. The total protein and free thyroxine (T4) levels were significantly higher in the pretreatment serum of the patients when compared to their posttreatment levels (P < 0.05) [Table - 2] and [Table - 3]. No significant differences were observed between before–after acitretin treatment in terms of thyroid-stimulating hormone, fT3, follicle-stimulating hormone, luteinizing hormone, prolactin, adrenocorticotropic hormone, growth hormone, parathyroid hormone, insulin-like growth factor binding-protein-3, homeostatic model assessment-insulin resistance, sex hormone-binding globulin, total testosterone, cortisol, insulin, estradiol and somatomedin C levels in psoriasis patients (P > 0.05) [Table - 1] and [Table - 3].
Discussion
In this study, no significant difference was found in pituitary hormone levels before and after acitretin treatment in patients with psoriasis, except free T4 levels. Therefore, acitretin treatment does not appear to affect endocrine production of the pituitary hormones being studied.
There are a very limited number of clinical studies in literature investigating the effects of retinoid therapy on pituitary hormone levels. The previous clinical studies investigating the effects of retinoid therapy on pituitary hormone levels are listed in [Table - 4].[17],[18],[19],[20],[21]
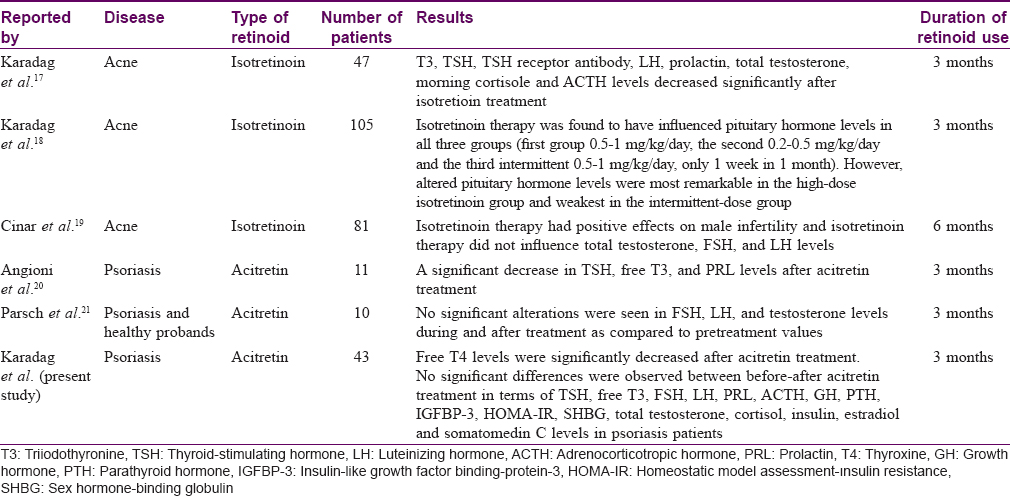
The present study found a significant decrease in free T4 levels after acitretin therapy; however, there was no significant change in other pituitary hormone levels.
Growth hormone and prolactin secreted by the pituitary gland are among the growth factors acting on keratinocytes.[22] However, studies which investigated growth hormone and prolactin levels in patients with psoriasis did not yield consistent results.[3],[22],[23],[24],[24],[25] A study reported higher prolactin levels in psoriasis patients, compared to the control group, decreased prolactin levels with the therapy, and a positive correlation between the disease severity and prolactin levels.[3] On the contrary, Sanlı Erdogan et al. reported no significant change in growth hormone and prolactin levels in patients with psoriasis, compared to the control group.[24] Similarly, Yavuz et al. did not find a significant difference in the prolactin levels between the patients with psoriasis and the control group.[25]
There are also studies examining the effects of other agents used in the treatment of psoriasis on pituitary hormone levels. A previous study investigated the effects of phototherapy, hydroxyurea, cyclosporine-A and acitretin therapy, all used in the treatment of psoriasis on somatotropic axis hormone levels.[11] The authors reported that not all therapies resulted in a significant change in growth hormone concentrations in active and remission phases of the disease; however, insulin-like growth factor binding-protein-3 levels were significantly higher in the phototherapy group, and insulin-like growth factor-1 levels were higher in the cyclosporine-A group during remission. Also, acitretin therapy did not cause significant changes in somatotropic axis hormones, similar to what we found in the present study.
Furthermore, vitamin A and its derivatives have been shown to have effects on distinct hormonal pathways in animal experiments.[13],[14],[15] Sharma et al. reported various effects of RXR selective retinoids on hypothalamic–pituitary–thyroid axis in mice including direct suppression of thyroid-stimulating hormone secretion.[13] Minegishi et al. showed that retinoic acid repressed follicle stimulating hormone-induced luteinizing hormone receptors in rat granulosa cells.[15]
The limitations of this study were a retrospective nature, inability to retest blood samples of 7 patients at 3 months post treatment onset, low dose and short duration of acitretin treatment. These limitations could have led to false negative results.
As a result, although many studies have been performed related to the etiopathogenesis of psoriasis, it is a disease with many unknown factors. We evaluated the effects of acitretin treatment on pituitary hormone levels in patients with psoriasis. We observed that acitretin treatment in patients with psoriasis did not affect the levels of pituitary hormones. More comprehensive and large-scale studies are needed to gain insight into the role of pituitary hormones in psoriasis pathogenesis and the effects of acitretin treatment on pituitary hormones.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Foitzik K, Langan EA, Paus R. Prolactin and the skin: A dermatological perspective on an ancient pleiotropic peptide hormone. J Invest Dermatol 2009;129:1071-87.
[Google Scholar]
|
| 2. |
Mowad CM, Margolis DJ, Halpern AC, Suri B, Synnestvedt M, Guzzo CA, et al. Hormonal influences on women with psoriasis. Cutis 1998;61:257-60.
[Google Scholar]
|
| 3. |
Dilmé-Carreras E, Martín-Ezquerra G, Sánchez-Regaña M, Umbert-Millet P. Serum prolactin levels in psoriasis and correlation with cutaneous disease activity. Clin Exp Dermatol 2011;36:29-32.
[Google Scholar]
|
| 4. |
Arican O, Bilgic K, Koc K. The effect of thyroid hormones in psoriasis vulgaris. Indian J Dermatol Venereol Leprol 2004;70:353-6.
[Google Scholar]
|
| 5. |
Zangeneh ZF, Fazeli A. The significance of stress hormones in psoriasis. Acta Med Iran 2008;46:485-8.
[Google Scholar]
|
| 6. |
Elias AN, Dangaran K, Barr RJ, Rohan MK, Goodman MM. A controlledtrial of topical propylthiouracil in the treatment of patients with psoriasis. J Am Acad Dermatol 1994;31:455-8.
[Google Scholar]
|
| 7. |
Elias AR, Barr RJ. Low-dose oral propylthiouracil in the treatment of plaque psoriasis. Int J Dermatol 1995;34:519-20.
[Google Scholar]
|
| 8. |
Evers AW, Verhoeven EW, Kraaimaat FW, de Jong EM, de Brouwer SJ, Schalkwijk J, et al. How stress gets under the skin: Cortisol and stress reactivity in psoriasis. Br J Dermatol 2010;163:986-91.
[Google Scholar]
|
| 9. |
Brunoni AR, Santos IS, Sabbag C, Lotufo PA, Benseñor IM. Psoriasis severity and hypothalamic-pituitary-adrenal axis function: Results from the CALIPSO study. Braz J Med Biol Res 2014;47:1102-6.
[Google Scholar]
|
| 10. |
Robati RM, Toossi P, Rahmati-Roodsari M, Khalilazar S, Abolhasani E, Namazi N, et al. Association of psoriasis severity with serum prolactin, thyroid hormones, and cortisol before and after treatment. Scientific World Journal 2013;2013:921819.
[Google Scholar]
|
| 11. |
Damasiewicz-Bodzek A, Kos-Kudła B, Suwała-Jurczyk B. The effect of various methods of psoriasis treatment on somatotrophin axis hormones in serum. J Clin Pharm Ther 2006;31:343-9.
[Google Scholar]
|
| 12. |
Lefebvre P, Martin PJ, Flajollet S, Dedieu S, Billaut X, Lefebvre B, et al. Transcriptional activities of retinoic acid receptors. Vitam Horm 2005;70:199-264.
[Google Scholar]
|
| 13. |
Sharma V, Hays WR, Wood WM, Pugazhenthi U, St. Germain DL, Bianco AC, et al. Effects of rexinoids on thyrotrope function and the hypothalamic-pituitary-thyroid axis. Endocrinology 2006;147:1438-51.
[Google Scholar]
|
| 14. |
Marissal-Arvy N, Hamiani R, Richard E, Moisan MP, Pallet V. Vitamin A regulates hypothalamic-pituitary-adrenal axis status in LOU/C rats. J Endocrinol 2013;219:21-7.
[Google Scholar]
|
| 15. |
Minegishi T, Hirakawa T, Kishi H, Abe K, Ibuki Y, Miyamoto K, et al. Retinoic acid (RA) represses follicle stimulating hormone (FSH)-induced luteinizing hormone (LH) receptor in rat granulosa cells. Arch Biochem Biophys 2000;373:203-10.
[Google Scholar]
|
| 16. |
Siegenthaler G, Gumowski-Sunek D, Saurat JH. Metabolism of natural retinoids in psoriatic epidermis. J Invest Dermatol 1990;95:47S-8S.
[Google Scholar]
|
| 17. |
Karadag AS, Ertugrul DT, Tutal E, Akin KO. Isotretinoin influences pituitary hormone levels in acne patients. Acta Derm Venereol 2011;91:31-4.
[Google Scholar]
|
| 18. |
Karadag AS, Takci Z, Ertugrul DT, Bilgili SG, Balahoroglu R, Takir M, et al. The effect of different doses of isotretinoin on pituitary hormones. Dermatology 2015;230:354-9.
[Google Scholar]
|
| 19. |
Çinar L, Kartal D, Ergin C, Aksoy H, Karadag MA, Aydin T, et al. The effect of systemic isotretinoin on male fertility. Cutan Ocul Toxicol 2016;35:296-9.
[Google Scholar]
|
| 20. |
Angioni AR, Lania A, Cattaneo A, Beck-Peccoz P, Spada A. Effects of chronic retinoid administration on pituitary function. J Endocrinol Invest 2005;28:961-4.
[Google Scholar]
|
| 21. |
Parsch EM, Ruzicka T, Przybilla B, Schill WB. Andrological investigations in men treated with acitretin (Ro 10-1670). Andrologia 1990;22:479-82.
[Google Scholar]
|
| 22. |
Sanchez Regana M, Umbert Millet P. Psoriasis in association with prolactinoma: three cases. Br J Dermatol 2000; 143:864-7.
[Google Scholar]
|
| 23. |
Giasuddin AS, El-Sherif AI, El-Ojali SI. Prolactin: does it have a role in the pathogenesis of psoriasis? Dermatology1998; 197:119-22.
[Google Scholar]
|
| 24. |
Sanlı Erdogan B, Aktan S, Ergin S, Gelincik N, Uz N. Serum levels of prolactin and growth hormone in patients with psoriasis. Turk Klin J Dermatol 2005;15:23-6.
[Google Scholar]
|
| 25. |
Yavuz Ö, Kavak A, Parlak AH, Anıl H, Aydogan I. The role of prolactin in psoriasis. Turkderm 2002;36:263-7.
[Google Scholar]
|
Fulltext Views
4,506
PDF downloads
3,267





