Translate this page into:
Effect of platelet counts and activator in platelet-rich plasma on the treatment of androgenetic alopecia, split-head comparison: A randomised, double-blind study
Corresponding author: Prof. Satyendra K Singh, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. drsatyendraderma@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh SK, Singh S. Effect of platelet counts and activator in platelet-rich plasma on the treatment of androgenetic alopecia, split head comparison: A randomised, double-blind study. Indian J Dermatol Venereol Leprol 2023;89:647-55
Abstract
Background
Androgenetic alopecia is a common, chronic, non-scarring alopecia. It is characterised by stepwise miniaturisation of the hair follicles, due to alteration in the hair cycle dynamics, leading to the transformation of terminal hair follicles into a vellus ones. Oral finasteride and topical minoxidil are the only approved drugs for treating this condition. Due to a limited number of effective therapies for androgenetic alopecia, platelet-rich plasma may be an effective alternative treatment.
Aims
To study the effect of activator in platelet-rich plasma and baseline platelet count in platelet-rich plasma on the treatment of androgenetic alopecia.
Methods
A randomised, double-blind split-head comparative study. The sample size was calculated and randomisation was done. Patients with androgenetic alopecia were allocated into two groups; in the first group, autologous activated platelet-rich plasma was injected in the right half of the affected scalp and autologous non-activated platelet-rich plasma was injected in the left half of the affected scalp and vice versa in the second group. Patients were also categorised on the basis of platelet counts in their platelet-rich plasma in three groups; group A (6-8 lakh/mm3), group B (8.1-10 lakh/mm3) and group C (>10 lakh/mm3). Interventions were done monthly for three months and followed up for the next three months. Effects of interventions were assessed by hair density, hair thickness, patient self-assessment and clinical photography.
Results
A total of 80 patients were included in the study. Activated platelet-rich plasma produced significant improvement of hair density after four months and hair thickness at 6 months. An increase in platelet count led to a significant increase in hair density and hair thickness after three and four months respectively and a highly significant increase in both parameters at the end of the study.
Limitations
Long-term follow-up of cases was not done and no measurement of vellus hair count was done.
Conclusion
There is a significant effect of activator and platelet count of the platelet-rich plasma on hair density as well as hair thickness.
Keywords
Androgenetic alopecia
platelet rich plasma
activator
platelet counts
randomised double blind
Plain Language Summary
Androgenetic alopecia (AGA) is a common, chronic, disease. AGA is characterized by thinning of hair. Oral finasteride and topical minoxidil are the only approved drugs. Due to limited number of effective therapies for AGA, platelet-rich plasma (PRP) may be an effective alternative treatment. We planned to see the effect of activator in PRP and baseline platelet count in PRP on treatment of AGA. PRP was prepared by collecting 25 cc blood into an autoclaved falcon tube. The tube was rotated in a centrifugation machine. Patients with AGA were allocated in two groups; in first group PRP with activator was injected in right half of the affected scalp and PRP without activator was injected in left half of affected scalp and vice versa in second group. Patients were also categorized on the basis of platelet counts in their PRP in three groups; group A (6-8 lakh/mm3), group B (8.1-10 lakh/mm3) and group C (>10 lakh/mm3). Interventions were done monthly for three months and the subjects followed up for the next three months. A total of 80 patients were included in the study. Effect of activator on hair density and hair thickness was significant improvement. Increase in platelet counts leads to increase in hair density and hair thickness.
Introduction
Androgenetic alopecia is a common, chronic, non-scarring form of alopecia that affects up to 80% of men and 50% of women during their lifetime.1 Significant impairment in quality of life is seen equally in males and females suffering from androgenetic alopecia.2 Topical minoxidil and oral finasteride are the only FDA-approved treatment for androgenetic alopecia.3
However, the efficacy of topical minoxidil and oral finasteride is often not very good and it only lasts as long as they are used. Several side effects with minoxidil (headache, irritation, itching, increased facial hair) and with finasteride (impotency, loss of libido) have been reported. There is a need for an alternative or additive treatment for androgenetic alopecia. One study,4 showed a lack of association between platelet counts, growth factors (platelet derived growth factor, epidermal growth factor and vascular endothelial growth factor) level and clinical improvement. Another study,5 showed patients treated with non-activated platelet-rich plasma had a greater increase in hair density than patients treated with activated platelet-rich plasma, but the limitation of the study was the small sample size, evaluation of hair density at different time intervals and that the platelet-rich plasma used in activation and non-activation was made by different methods.
Hence, we planned a randomised, double-blind split-head comparative study to see the effect of activator and platelet counts in platelet-rich plasma for the treatment of androgenetic alopecia.
Methods
Study design
A single-centre, prospective, randomised, double-blind split-head comparative study. Informed written consent was taken. Institution Ethics Committee approval was taken for the protocol submitted and registered with the Clinical Trials Registry of India (CTRI/2019/05/019092). The study was conducted from May 2019 to June 2020.Inclusion criteria were 20-50 years male patients with androgenetic alopecia [Hamilton-Norwood scale (HNS) II-VI], not using topical minoxidil and oral or topical finasteride within six months and willing to come for regular follow-up.Exclusion criteria were those having any inflammation or erythema (except mild seborrheic dermatitis), or scarring over the scalp. Patients taking anticoagulants, antihypertensives, anticonvulsants, aspirin or other nonsteroidal anti-inflammatory drugs. Patients with any of the following were also excluded: A current or previous history of malignancies, platelet or bleeding disorders, bone marrow aplasia, diabetes, HIV, hepatitis B or C infection or otherwise immunocompromised, those having a propensity for keloids, the concomitant presence of other causes of hair loss such as alopecia areata, acute and chronic telogen effluvium and hair loss of chronic illness.
Sample size calculation
It was observed from the previous study,5 the mean ± standard deviation value of hair density at baseline with platelet-rich plasma without activator was 218 ± 17 and with activator was 160 ± 6 and after six months’ follow-up after treatment, these values were 282 ± 20 and 250 ± 12, respectively. Taking the power of the study is 90%, the sample size would be 74 and assuming the 10% dropout during follow-up, the required sample size would be 82.
Diagnosis of male type baldness
On the basis of a detailed medical history of diffuse hair loss, clinical examination and dermoscopic features (miniaturisation and more than 20% variability in hair diameter between affected and uninvolved areas). Androgenetic alopecia grading was done according to the Hamilton-Norwood scale.
Platelet-rich plasma preparation
Platelet-rich plasma was prepared by collecting 25 cc of fresh whole blood mixed with 3 cc of 28% sodium citrate solution into an autoclaved falcon tube under proper aseptic precautions. The tube was rotated in a centrifugation machine (Remi model R-8M) at 1400 revolutions per minute for 15 minutes. The first centrifugation allows blood separation into three layers, namely the bottom RBC layer, the topmost acellular plasma layer called platelet-poor plasma, and an intermediate platelet-rich plasma layer called the “buffy coat.” The buffy coat along with plasma was collected with the help of finnipipette into another falcon tube. This tube underwent second centrifugation, which was faster than the first, comprising at 2200 revolutions per minute for 17 minutes. This allowed the platelets to settle at the bottom of the tube, which is platelet-rich plasma. The upper 2/3rd containing platelet-poor plasma was discarded and the lower 1/3rd of platelet-rich plasma was injected in the area earmarked for non-activated platelet-rich plasma. For the area where activated platelet-rich plasma had to be injected, this platelet-rich plasma was loaded in an insulin syringe containing calcium gluconate 10% (one-part calcium gluconate 10% and nine parts of platelet-rich plasma) as an activator. With this method, we achieved 4-5 mL of platelet-rich plasma which had a platelet concentration of 3-5 folds as compared to the whole blood. The volume of platelet-rich plasma given in one sitting was 2 mL on both activated and non-activated sites in all patients. The scalp affected by hair loss was cleaned with 70% alcohol. Platelet-rich plasma (0.05-0.1 mL/cm2 or 2-4 units by insulin syringe) was injected intra-dermally in a retrograde fashion from deep to superficial, at a distance of one centimetre. Injection of platelet-rich plasma was given three times in each patient at intervals of one month.
Randomisation
The allocation sequence was generated using an online randomisation generator using the website and concealed by a person unrelated to the trial management group. Only one person who was introducing the drugs had knowledge about the randomisation of the subject. The patient, evaluator and analyser had no knowledge of the randomisation group.
Cases were randomised into two groups:
Group 1: platelet-rich plasma (0.1 mL/cm2) was injected into the right half of the affected scalp with an activator (AA PRP) and the left half of the affected scalp without activator.
Group 2: platelet-rich plasma (0.1 mL/cm2) was injected into the left half of the affected scalp with an activator (AA PRP) and the left half of the affected scalp without activator.
Patients were also categorised on the basis of platelet counts in their platelet-rich plasma in three groups:
Group A: 6-8 lakh/mm3
Group B: 8.1-10 lakh/mm3
Group C: >10 lakh/mm3
Three injections of platelet-rich plasma were given at baseline, after one month and two months.
Patient assessment
All patients were evaluated: before treatment (BT); at one month (F1); at two months (F2), at three months (F3), at four months (F4), at five months (F5) and six months of intervention (F6). The effect of the treatment was assessed by hair density, hair diameter, patient self-assessment and clinical photography documented every month for six months.The primary outcomes were hair density and hair thickness.
The secondary outcomes were patient self-assessment and changes in the Norwood Hamilton scale.
Assessment of hair density and hair thickness was done by dermoscopic pictures taken at a fixed site in a 1-cm2 area. The assessment area was fixed at 10 cm above and 3 cm in front from the upper end of the tragus. A stamp of 1 × 1 cm2 was used to fix the area, and every month dermoscopic pictures were taken from this area and with the help of ImageJ software and hair thickness was calculated.
Patient self-assessment score
Patients assessed their scalp hair on a hair growth assessment scale of 0-4.
0: No improvement
1: mild (1-25%).
2: moderate (26-50%).
3: marked (>50%).
Clinical photographs were taken at every session from the front, vertex and lateral views of both sides.
Assessment of improvement in HNS: (only for this study)
Worsening.
Unchanged.
Mild improvement-If there was 1 stage improvement in HNS staging, e.g., V to IV, IV to III, III to II, etc.).
Moderate improvement- If there was two-stage improvement in HNS staging, e.g., V to III, IV to II, etc.
Marked improvement- If there was three-stage or higher improvement in HNS staging, e.g., V to II, IV to I, etc.
Statistical analysis
The data obtained were analysed by SPSS 23.0 trial version. The data were presented in terms of means, standard deviation and percentages. To assess the association between categorical variables, “Chi-square test” was used as per suitability, to find out the effect of intervention after uninterrupted completion of six months’ study, paired t-test and analysis of variance were used and to find out the effect of intervention in two areas unpaired t-test was used. A P-value less than 0.05 was taken as statistically significant.
Results
A total of 315 patients were screened and finally 86 patients were included in the study (Flowchart 1)
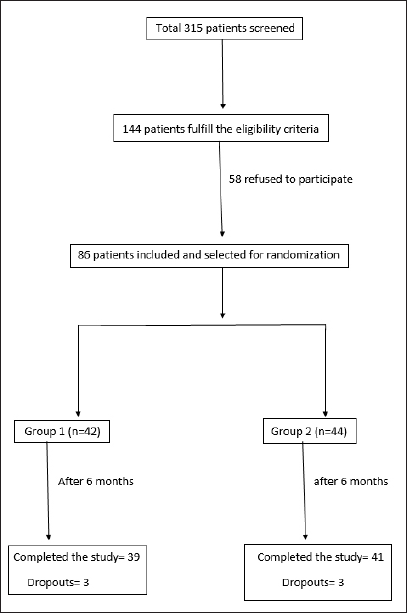
- Patient screening and selection
Patient characteristics are given in Table 1.
| Total no. of cases | 80 |
|---|---|
| Ages (years): | |
| Range | 21–40 |
| Mean ± SD | 26.14 ± 3.9 |
| Age of onset (years): | |
| Range | 19–32 |
| Mean ± SD | 22.34 ± 5.8 |
| Duration: | |
| <1 year | 24 |
| 1.1–5 years | 44 |
| 5.1–10 years | 8 |
| >10 years | 4 |
| Marital status: | |
| Married | 8 |
| Unmarried | 72 |
| Educational status: | |
| Upto high school | 4 |
| Intermediate | 12 |
| Graduate | 64 |
| Occupation: | |
| Student | 52 |
| Businessman | 12 |
| Service | 16 |
| Hamilton Norwood scale: | |
| Stage II | 11 |
| Stage III | 29 |
| Stage IIIa | 1 |
| Stage IIIv | 8 |
| Stage IV | 15 |
| Stage IVa | 0 |
| Stage IVv | 5 |
| Stage V | 7 |
| Stage Va | 0 |
| Stage VI | 4 |
The effect of the activator on hair density is given in Table 2.
| Intervention | Hair density | Mean difference ± SD, percentage, P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | F1 | F2 | F3 | F4 | F5 | F6 | F1-BT | F2-BT | F3-BT | F4-BT | F5-BT | F6-BT | |
| Area with activator (n = 80) | 90.05 ± 11.47 | 91.69 ± 11.54 | 94.28 ± 11.83 | 99.92 ± 12.58 | 106.10 ± 13.80 | 112.05 ± 14.86 | 117.80 ± 16.20 | 1.63 ± 0.76 1.81% P < 0.001 |
4.22 ± 1.75 4.69% P < 0.001 |
9.87 ± 4.14 10.96% P < 0.001 |
16.05 ± 6.70 17.82% P < 0.001 |
22.00 ± 8.85 24.43% P < 0.001 |
27.75 ± 11.38 30.81% P < 0.001 |
| Area without activator (n = 80) | 89.60 ± 11.44 | 91.00 ± 11.41 | 92.86 ± 11.49 | 96.62 ± 11.81 | 101.48 ± 12.27 | 106.76 ± 13.39 | 112.71 ± 14.64 | 1.40 ± 0.70 1.56% P < 0.001 |
3.26 ± 1.43 3.64% P < 0.001 |
7.02 ± 3.51 7.84% P < 0.001 |
11.87 ± 5.23 13.25% P < 0.001 |
17.16 ± 7.24 19.15% P < 0.001 |
23.11 ± 9.63 25.79% P < 0.001 |
| Mean difference | 0.45 | 0.69 | 1.41 | 3.30 | 4.63 | 5.29 | 5.08 | ||||||
| P-value | 0.80 | 0.70 | 0.44 | 0.08 | <0.05 | <0.05 | <0.05 | ||||||
| Intervention | Hair thickness | Mean difference ± SD, percentage, P-value | |||||||||||
| BT | F1 | F2 | F3 | F4 | F5 | F6 | F1-BT | F2-BT | F3-BT | F4-BT | F5-BT | F6-BT | |
| Area with activator (n = 80) | 59.96 ± 9.96 | 60.90 ± 9.99 | 62.48 ± 10.03 | 64.94 ± 10.00 | 68.31 ± 10.32 | 71.72 ± 10.53 | 75.30 ± 10.99 | 0.93 ± 0.73 1.56% P < 0.001 |
2.51 ± 1.35 4.19% P < 0.001 |
4.97 ± 2.15 8.29% P < 0.001 |
8.35 ± 3.47 13.92% P < 0.001 |
11.76 ± 4.58 19.61% P < 0.001 |
15.33 ± 6.13 25.57% P < 0.001 |
| Area without activator (n = 80) | 59.75 ± 9.98 | 60.58 ± 9.97 | 61.65 ± 9.90 | 63.25 ± 9.89 | 65.64 ± 9.89 | 68.72 ± 9.96 | 71.55 ± 10.38 | 0.82 ± 0.63 1.38% P < 0.001 |
1.90 ± 1.17 3.18% P < 0.001 |
3.50 ± 1.65 5.85% P < 0.001 |
5.88 ± 2.76 9.85% P < 0.001 |
8.97 ± 3.65 15.02% P < 0.001 |
11.80 ± 4.73 19.74% P < 0.001 |
| Mean difference | 0.21 | 0.33 | 0.82 | 1.69 | 2.68 | 3.00 | 3.75 | ||||||
| P-value | 0.89 | 0.83 | 0.60 | 0.28 | 0.09 | 0.06 | <0.05 | ||||||
BT: before treatment; F1: at 1 month; F2: at 2 months, F3: at 3 months, F4: at 4 months, F5: at 5 months and F6: 6 months of intervention
In inter-area comparison, a statistically significant difference in hair density (P < 0.05) was seen at F4, F5 and F6. In intra-area comparison, in both areas with activators and areas without activator, there was a highly significant difference (P < 0.001) in hair density at each visit.
The effect of the activator on hair thickness is given in Table 2.
In inter-area comparison, a statistically significant difference (P < 0.05) in hair thickness was seen at the end of the study.
In intra-area comparison, in both areas with activator and areas without activator, there was a highly significant difference (P < 0.001) in hair thickness at each visit.
In the inter-group comparison of the effect of platelet counts on hair density, there was a significant difference in hair density (P < 0.05) between groups at F3 and a highly significant difference (P < 0.001) at F4, F5 and F6. In intra-group comparison, there was a highly significant difference (P < 0.001) in hair density in each group at each visit. At six months, increase in hair density was: group C > B >A (42%, 34% and 16%) [Table 3].
| Groups | Hair density | Mean difference ± SD, percentage, P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | F1 | F2 | F3 | F4 | F5 | F6 | F1-BT | F2-BT | F3-BT | F4-BT | F5-BT | F6-BT | |
| Group A (n = 28) | 90.18 ± 11.65 | 91.25 ± 11.58 | 92.79 ± 11.38 | 95.54 ± 11.13 | 98.50 ± 10.78 | 101.79 ± 10.90 | 104.57 ± 10.72 | 1.07 1.18% P < 0.001 |
2.60 2.89% P < 0.001 |
5.35 5.94% P < 0.001 |
8.32 9.22% P < 0.001 |
11.60 12.87% P < 0.001 |
14.39 15.96% P < 0.001 |
| Group B (n = 21) | 87.00 ± 13.68 | 89.19 ± 13.94 | 92.05 ± 14.43 | 99.00 ± 14.69 | 105.62 ± 15.25 | 111.43 ± 14.46 | 116.95 ± 13.58 | 2.19 2.51% P < 0.001 |
5.04 5.80% P < 0.001 |
12.00 13.79% P < 0.001 |
18.61 21.40% P < 0.001 |
24.42 28.07% P < 0.001 |
29.95 34.42% P < 0.001 |
| Group C (n = 31) | 92.00 ± 9.48 | 93.77 ± 9.56 | 97.13 ± 9.99 | 104.52 ± 11.01 | 113.29 ± 11.58 | 121.74 ± 11.82 | 130.32 ± 11.79 | 1.77 1.92% P < 0.001 |
5.12 5.57% P < 0.001 |
12.51 13.64% P < 0.001 |
21.29 23.14% P < 0.001 |
29.74 32.32% P < 0.001 |
38.32 41.65% P < 0.001 |
| Total (n = 80) | 90.05 ± 11.47 | 91.69 ± 11.54 | 94.28 ± 11.83 | 99.93 ± 12.58 | 106.10 ± 13.80 | 112.05 ± 14.86 | 117.80 ± 16.20 | 1.63 1.81% P < 0.001 |
4.22 4.69% P < 0.001 |
9.87 10.96% P < 0.001 |
16.05 17.82% P < 0.001 |
22.00 24.43% P < 0.001 |
27.75 30.81% P < 0.001 |
| F-value | 1.19 | 1.01 | 1.51 | 4.12 | 10.5 | 19.49 | 34.30 | ||||||
| P-value | 0.31 | 0.36 | 0.22 | <0.05 | <0.001 | <0.001 | <0.001 | ||||||
| Post hoc test | |||||||||||||
| A vs B | P = 0.33 | P = 0.53 | P = 0.82 | P = 0.32 | P < 0.05 | P < 0.05 | P < 0.001 | ||||||
| A vs C | P = 0.54 | P = 0.40 | P = 0.16 | P = 0.05 | P < 0.001 | P < 0.001 | P < 0.001 | ||||||
| B vs C | P = 0.12 | P = 0.16 | P = 0.13 | P = 0.11 | P < 0.05 | P < 0.05 | P < 0.001 | ||||||
| Groups | Hair thickness | Mean difference ± SD, percentage, P-value | |||||||||||
| BT | F1 | F2 | F3 | F4 | F5 | F6 | F1-BT | F2-BT | F3-BT | F4-BT | F5-BT | F6-BT | |
| Group A (n = 28) | 61.21 ± 9.47 | 61.75 ± 9.36 | 62.86 ± 9.34 | 64.61 ± 9.15 | 66.50 ± 9.24 | 68.54 ± 9.22 | 70.82 ± 9.51 | 0.53 0.87% P < 0.001 |
1.64 2.68% P < 0.001 |
3.39 5.54% P < 0.001 |
5.28 8.63% P < 0.001 |
7.32 11.96% P < 0.001 |
9.60 15.69% P < 0.001 |
| Group B (n = 21) | 56.19 ± 12.55 | 57.48 ± 12.78 | 58.86 ± 12.35 | 61.29 ± 12.32 | 65.19 ± 12.66 | 69.00 ± 12.60 | 72.71 ± 12.77 | 1.28 2.28% P < 0.001 |
2.66 4.74% P < 0.001 |
5.09 9.06% P < 0.001 |
9.00 16.01% P < 0.001 |
12.81 22.79% P < 0.001 |
16.52 29.40% P < 0.001 |
| Group C (n = 31) | 61.39 ± 7.88 | 62.45 ± 7.99 | 64.58 ± 8.42 | 67.71 ± 8.33 | 72.06 ± 8.52 | 76.45 ± 8.48 | 81.10 ± 8.36 | 1.06 1.73% P < 0.001 |
3.19 5.20% P < 0.001 |
6.32 10.30% P < 0.001 |
10.67 17.39% P < 0.001 |
15.06 24.54% P < 0.001 |
19.71 32.10% P < 0.001 |
| Total (n = 80) | 59.96 ± 9.96 | 60.90 ± 9.99 | 62.48 ± 10.03 | 64.94 ± 10.00 | 68.31 ± 10.32 | 71.73 ± 10.53 | 75.30 ± 10.99 | 0.93 1.56% P < 0.001 |
2.51 4.19% P < 0.001 |
4.97 8.29% P < 0.001 |
8.35 13.92% P < 0.001 |
11.76 19.61% P < 0.001 |
15.33 25.57% P < 0.001 |
| F-value | 1.19 | 1.73 | 2.12 | 2.71 | 3.66 | 5.71 | 8.59 | ||||||
| P value | 0.30 | 0.18 | 0.12 | 0.07 | <0.05 | <0.05 | <0.001 | ||||||
| Post hoc test | |||||||||||||
| A Vs B | P = 0.08 | P = 0.13 | P = 0.16 | P = 0.24 | P < 0.65 | P < 0.87 | P < 0.51 | ||||||
| A Vs C | P = 0.94 | P = 0.78 | P = 0.50 | P = 0.22 | P < 0.05 | P < 0.05 | P < 0.001 | ||||||
| B Vs C | P = 0.06 | P = 0.07 | P = 0.05 | P = 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | ||||||
BT: before treatment; F1: at 1 month; F2: at 2 months, F3: at 3 months, F4: at 4 months, F5: at 5 months and F6: 6 months of intervention; Group A: patients with platelet counts 6-8 lakh/mm3; Group B: patients with platelet counts 8.1-10 lakh/mm3; Group C: patients with platelet counts >10 lakh/mm3
The effect of platelet counts on hair thickness is given in Table 3, which shows a significant difference (P < 0.05) between groups at F4 and F5 and a highly significant difference (P < 0.001) at F6. In intra-group comparison, there was a highly significant difference (P < 0.001) in hair thickness in each group at each visit. At six months, increase in hair thickness was: group C > B > A (32%, 29% and 16%) Figures 1 & 2.
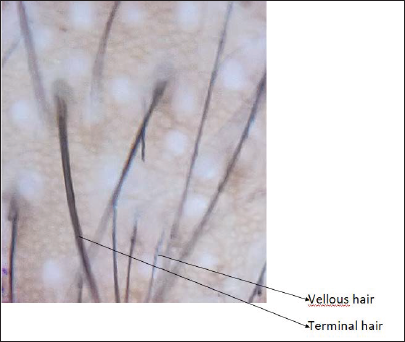
- Dermoscopic image before treatment (magnification ×910)
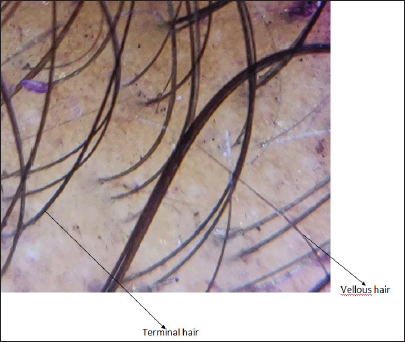
- Dermoscopic image after six months (magnification ×910)
Age and NHS did not affect the response [Table 4].
| Age group(s) | Hair density (hair count/cm2) | Mean difference ± SD, percentage and P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | F1 | F2 | F3 | F4 | F5 | F6 | F1-BT | F2-BT | F3-BT | F4-BT | F5-BT | F6-BT | |
| 20-30 years (n = 62) | 90.84 ± 11.31 | 92.47 ± 11.39 | 95.03 ± 11.65 | 100.50 ± 12.48 | 106.77 ± 13.52 | 112.68 ± 14.48 | 118.48 ± 15.74 | 1.62 ± 0.79 1.79% P < 0.001 |
4.19 ± 1.87 4.61% P < 0.001 |
9.66 ± 4.24 10.64% P < 0.001 |
15.93 ± 6.82 17.54% P < 0.001 |
21.83 ± 8.93 24.04% P < 0.001 |
27.64 ± 11.52 30.43% P < 0.001 |
| 31-40 years (n = 18) | 87.33 ± 11.92 | 89.00 ± 12.00 | 91.67 ± 12.42 | 97.94 ± 13.10 | 103.78 ± 14.90 | 109.89 ± 16.38 | 115.44 ± 17.99 | 1.66 ± 0.68 1.90% P < 0.001 |
4.33 ± 1.28 4.96% P < 0.001 |
10.61 ± 3.82 12.15% P < 0.001 |
16.44 ± 6.42 18.83% P < 0.001 |
22.55 ± 8.80 25.83% P < 0.001 |
28.11 ± 11.19 32.18% P < 0.001 |
| P value | 0.85 | 0.76 | 0.39 | 0.77 | 0.76 | 0.88 | |||||||
| Age group | Hair thickness (μm) | Mean difference ± standard deviation, percentage and P-value | |||||||||||
| BT | F1 | F2 | F3 | F4 | F5 | F6 | F1-BT | F2-BT | F3-BT | F4-BT | F5-BT | F6-BT | |
| 20-30 years (n = 62) | 60.94 ± 9.65 | 61.87 ± 9.68 | 63.40 ± 9.72 | 65.85 ± 9.76 | 69.18 ± 10.02 | 72.56 ± 10.26 | 76.19 ± 10.75 | 0.93 ± 0.78 1.54% P < 0.001 |
2.46 ± 1.38 4.05% P < 0.001 |
4.88 ± 2.22 8.02% P < 0.001 |
8.24 ± 3.61 13.52% P < 0.001 |
11.62 ± 4.77 19.08% P < 0.001 |
15.25 ± 6.42 25.04% P < 0.001 |
| 31-40 years (n = 18) | 56.61 ± 10.56 | 57.56 ± 10.60 | 59.28 ± 10.69 | 61.89 ± 10.53 | 65.33 ± 11.08 | 68.83 ± 11.24 | 72.22 ± 11.56 | 0.94 ± 0.53 1.67% P < 0.001 |
2.66 ± 1.28 4.71% P < 0.001 |
5.27 ± 1.90 9.32% P < 0.001 |
8.72 ± 3.00 15.41% P < 0.001 |
12.22±3.94 21.59% P < 0.001 |
15.61 ± 5.20 27.58% P < 0.001 |
| P-value | 0.96 | 0.58 | 0.50 | 0.60 | 0.63 | 0.83 | |||||||
| HNS group | Hair density | Mean difference ± standard deviation, percentage and P-value | |||||||||||
| BT | F1 | F2 | F3 | F4 | F5 | F6 | F1-BT | F2-BT | F3-BT | F4-BT | F5-BT | F6-BT | |
| II and III (n = 49) | 97.08 ± 6.00 | 98.73 ± 6.21 | 101.43 ± 6.6 | 107.08 ± 7.96 | 113.29 ± 9.94 | 118.94 ± 11.52 | 124.20 ± 13.85 | 1.65 ± 0.83 1.70% P < 0.001 |
4.34 ± 2.01 4.48% P < 0.001 |
10.00 ± 4.56 10.30% P < 0.001 |
16.20 ± 7.36 16.69% P < 0.001 |
21.85 ± 9.41 22.51% P < 0.001 |
27.12 ± 12.20 27.94% P < 0.001 |
| IV, V and VI (n = 31) | 78.94 ± 8.98 | 80.55 ± 8.94 | 82.97 ± 9.14 | 88.61 ± 10.00 | 94.74 ± 11.22 | 101.16 ± 13.03 | 107.68 ± 14.55 | 1.61 ± 0.67 2.04% P < 0.001 |
4.03 ± 1.22 5.11% P < 0.001 |
9.67 ± 3.45 12.26% P < 0.001 |
15.80 ± 5.62 20.0% P < 0.001 |
22.22 ± 8.04 28.16% P < 0.001 |
28.74 ± 10.06 36.41% P < 0.001 |
| P value | 0.82 | 0.43 | 0.73 | 0.79 | 0.85 | 0.53 | |||||||
| HNS group | Hair thickness (μm) | Mean difference± standard deviation, percentage and P value | |||||||||||
| BT | F1 | F2 | F3 | F4 | F5 | F6 | F1-BT | F2-BT | F3-BT | F4-BT | F5-BT | F6-BT | |
| II and III (n = 49) | 66.43 ± 4.44 | 67.35 ± 4.64 | 68.88 ± 5.02 | 71.18 ± 5.33 | 74.39 ± 6.27 | 77.61 ± 6.97 | 80.90 ± 8.27 | 0.91 ± 0.88 1.38% P < 0.001 |
2.44 ± 1.56 3.69% P < 0.001 |
4.75 ± 2.47 7.16% P < 0.001 |
7.95 ± 3.90 11.98% P < 0.001 |
11.18 ± 5.02 16.84% P < 0.001 |
14.46 ± 6.67 21.78% P < 0.001 |
| III, IV and VI (n = 31) | 49.74 ± 7.33 | 50.71 ± 7.32 | 52.35 ± 7.21 | 55.06 ± 7.34 | 58.71 ± 7.90 | 62.42 ± 8.27 | 66.45 ± 8.77 | 0.96 ± 0.41 1.95% P < 0.001 |
2.61 ± 0.95 5.25% P < 0.001 |
5.32 ± 1.49 10.70% P < 0.001 |
8.96 ± 2.59 18.03% P < 0.001 |
12.67 ± 3.67 25.49% P < 0.001 |
16.71 ± 4.98 33.59% P < 0.001 |
| P-value | 0.77 | 0.60 | 0.25 | 0.20 | 0.15 | 0.11 | |||||||
BT: before treatment; F1: at 1 month; F2: at 2 months, F3: at 3 months, F4: at 4 months, F5: at 5 months and F6: 6 months of intervention
There was no significant difference in change in Norwood Hamilton scale at the end of the study in early (NHS II and III) and late (NHS IV, V and VI) grade of androgenetic alopecia [Table 5].
| Groups | No. of cases | Unchanged | Improvement after 6 months | Worsening | ||
|---|---|---|---|---|---|---|
| Mild | Moderate | Marked | ||||
| HNS III and IV | 58 | 7 (12%) | 28 (48.2%) | 17 (29.3%) | 4 (6.8%) | 2 (3.4%) |
| HNS V and VI | 11 | 2 (18%) | 4 (36.3%) | 2 (18%) | 2 (18%) | 1 (9%) |
| Chi-square value = 2.99 df = 4 P-value = 0.599 |
||||||
HNS: Hamilton-Norwood scale
Patient self-assessment is given in Table 6, which showed moderate to marked improvement in about 3/4th of patients Figures 3 & 4.
| Months | No improvement | Mild improvement | Moderate improvement | Marked improvement | Worsening |
|---|---|---|---|---|---|
| 1 | 6 (7.5%) | 27 (33.7%) | 40 (50%) | 5 (6.2%) | 2 (2.5%) |
| 2 | 4 (5%) | 26 (32.5%) | 42 (52.5%) | 7 (8.7%) | 1 (1.2%) |
| 3 | 2 (2.5%) | 28 (35%) | 41 (51.2%) | 9 (11.2%) | 0 |
| 4 | 1 (1.2%) | 22 (27.5%) | 44 (55%) | 13 (16.2%) | 0 |
| 5 | 0 | 24 (30%) | 42 (52.5%) | 12 (15%) | 2 (2.5%) |
| 6 | 2 (2.5%) | 16 (20%) | 44 (55%) | 15 (18.7%) | 3 (3.7%) |
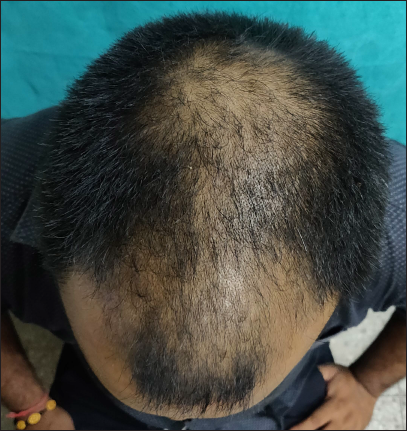
- Clinical image of androgenetic alopecia (Norwood Hamilton scale 4v) before treatment
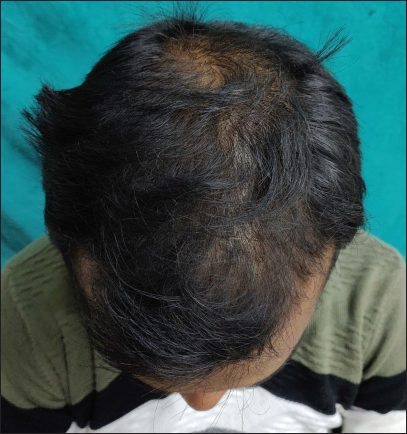
- Clinical image after six months of treatment
Discussion
Androgenetic alopecia not only affects beauty but also results in lower self-esteem, sadness, depression, stress and impaired quality of life.6,7 These problems are more pronounced in younger patients with more advanced alopecia.
Platelet-rich plasma is an autologous product of blood with a platelet concentration 3-8 times more than one found in the blood. Platelets recruited to the site of injury release proteins from their α-granules that display a wide range of functional properties from clot induction (adhesive proteins) and platelet aggregation (membrane glycoproteins) to vascularisation (cytokines and chemokines) and cell proliferation and differentiation (growth factors).8 Moreover, activated platelet release of the nucleotide adenosine diphosphate from dense granules activates additional platelets.9 Many of the growth factors released from the α-granules of activated platelets have been shown to have a positive impact on hair growth. Specifically, platelet-derived growth factor AA improves the hair inductive activity of dermal papilla cells when applied in combination with fibroblast growth factor 2.10 Insulin-like growth factor-1 stimulates proliferation of cycling Ki67+ basal keratinocytes,11,12 while transforming growth factor β1 protects the proliferative potential of basal keratinocytes by inhibiting cell growth and terminal differentiation.13,14 Vascular endothelial growth factor promotes angiogenesis.
In Gentile et al.5; study, two different clinical protocols in which one population (18 participants) received half-head treatment with autologous non-activated platelet-rich plasma (A-PRP) and the other half-head with placebo, and a second separated population in which all participants (n = 6, 3 participants per group) received treatment with calcium-activated platelet-rich plasma (AA-PRP) produced from one of two different platelet-rich plasma collection devices. In our study (80 participants) received half-head treatment with A-PRP and the other half-head with AA-PRP by manually prepared by Remi 8M centrifugation machine (Remiworld, India). Gentile et al.; administered three treatments at 30 days’ interval for the A-PRP group and a single injection for the AA-PRP group. They showed a clinical improvement in hair density in target area +65 ± 5 hairs/ cm2 (31%), three months’ post-treatment in A-PRP group and, hair density by +90 ± 6 hairs/cm2 (56%) when Arthrex Angel system was used but decreased in hair density by 73 ± 30 hairs/cm2 (23%) when Regen Blood cell therapy tube was used after six months of treatment in AA-PRP group. Hair thickness was not assessed in that study. In the present study both A-PRP and AA-PRP areas received three treatments at one-month intervals, dermoscopic analysis (DermLite DL4) and ImageJ software were used to assess the hair density and hair thickness at baseline and every month for six months. After six months, increase in hair density was found in both A-PRP and AA-PRP by 23 ± 9 hair cm2 (25.7%) and 27 ± 11 hair cm2 (30.8%) respectively and also increase in hair thickness in both A-PRP and AA-PRP by 11 ± 4 hair cm2 (19.7%) and 15 ± 6 hair cm2 (25.5%), respectively.
Rodrigues et al.4 used autologous serum as an activator, the study on 26 patients with androgenetic alopecia-III vertex, four subcutaneous injections of platelet-rich plasma were given and after three months of last injection, there was a significant increase in hair density and percentage of anagen hairs in platelet-rich plasma group.
Takikawa et al15 found six times platelet concentration in platelet-rich plasma using double spin centrifugation. The outcome of that study showed that platelet-rich plasma and normal saline injection did not increase the number of hairs in patients, but only increased the hair thickness. There was no increase in the number of hairs which may be due to the non-addition of the activator. The authors suggested that adding a carrier to platelet-rich plasma could increase the platelet-rich plasma effect.
Singh et al16 used calcium gluconate 10% as activator, they found platelet concentration of 4.2 times in platelet-rich plasma after double spin centrifugation. Three sessions of platelet-rich plasma were given monthly and after five months there was increase in hair density by 49 ± 5 hairs/cm2 (54%).
In present study, onset of action in relation to increase in hair density and hair thickness was noted even after one month of first platelet-rich plasma therapy with and without activator and with different platelet counts. Significant increase in hair density and hair thickness was found in both areas with activator and without activator at the end of the study. Effect of platelet counts on hair density and thickness was studied only with activated platelet-rich plasma. Both these outcome measures were found to be increased with higher platelet counts in the platelet-rich plasma. Effect of activator on hair density was statistically significant after four months of intervention and on hair thickness, at the end of the study (six months).
Temporary pain was reported by all patients after platelet-rich plasma treatment. Folliculitis was not found in any of the cases after platelet-rich plasma treatment in our study, similar to other studies. There was no major adverse effect in any of the patients.
Limitations
Long-term follow up of cases was not done, hair counts and hair thickness were not done over different parts of the scalp and measurement of vellus hair count and effect of treatment on anagen and telogen hair was not done. Trichoscan or folliscope were not used hence quality of dermoscopic pictures was not very good.
Conclusion
There is a significant effect of activator on hair density as well as on hair thickness. Increased platelet count in platelet-rich plasma causes significantly more improvement in terms of hair density and hair thickness.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- The psychosocial impact of hair loss among men: A multinational European study. Curr Med Res Opin. 2005;21:1829-36.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J Am Acad Dermatol. 2019;80:694-700.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of not-activated and activated PRP in hair loss treatment: Role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18:408.
- [CrossRef] [PubMed] [Google Scholar]
- The psychological effects of androgenetic alopecia in men. J Am Acad Dermatol. 1992;26:926-31.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137-9.
- [CrossRef] [PubMed] [Google Scholar]
- Platelets inflammation and tissue regeneration. Thromb Haemost. 2011;105:S13-33.
- [CrossRef] [PubMed] [Google Scholar]
- Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927-9.
- [CrossRef] [PubMed] [Google Scholar]
- Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro. J Dermatol Sci. 2015;79:110-8.
- [CrossRef] [PubMed] [Google Scholar]
- The insulin-like growth factor 1 receptor is expressed by epithelial cells with proliferative potential in human epidermis and skin appendages: Correlation of increased expression with epidermal hyperplasia. J Investig Dermatol. 1996;106:564-70.
- [CrossRef] [PubMed] [Google Scholar]
- Basic fibroblast growth-factor and insulin-like growth factor-I are strong mitogens for cultured mouse keratinocytes. J Cell Physiol. 1988;137:277-84.
- [CrossRef] [PubMed] [Google Scholar]
- Modulation of growth and differentiation in normal human keratinocytes by transforming growth-factor-β. J Cell Physiol. 1990;145:95-101.
- [CrossRef] [PubMed] [Google Scholar]
- Reversible inhibition of normal human prokeratinocyte proliferation by type-β transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986;46:2068-71.
- [PubMed] [Google Scholar]
- Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37:1721-9.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: A randomized, double-blind placebo control trial. Indian J Dermatol Venereol Leprol. 2020;86:150-7.
- [CrossRef] [PubMed] [Google Scholar]






