Translate this page into:
Effectiveness and safety analysis of rituximab in 146 Indian pemphigus patients: A retrospective single-center review of up to 68 months follow-up
2 Care India Solutions for Sustainable Development, Bihar Technical Support Program, Patna, Bihar, India
Correspondence Address:
Dipankar De
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh - 160 012
India
| How to cite this article: De D, Bishnoi A, Handa S, Mahapatra T, Mahajan R. Effectiveness and safety analysis of rituximab in 146 Indian pemphigus patients: A retrospective single-center review of up to 68 months follow-up. Indian J Dermatol Venereol Leprol 2020;86:39-44 |
Abstract
Background: Rituximab is being increasingly used for the treatment of pemphigus. Data derived from single-center studies following a uniform treatment protocol are limited. Effect of demography and disease type on treatment response is poorly characterized.
Objective: Our aim was to assess the effectiveness of biosimilar rituximab in pemphigus patients who had received rituximab as per rheumatoid arthritis protocol (2 doses, 1g each, infused 14 days apart).
Methods: It was a retrospective review of 146 eligible patients to assess the proportion of patients achieving complete remission off treatment, time to achieve complete remission off treatment, proportion of patients who relapsed after achieving complete remission off treatment, time taken to relapse, duration and total cumulative dose of corticosteroids administered after rituximab. Additionally, we tried to find whether a correlation existed between age, gender, total duration of illness before rituximab and pemphigus disease type with the above-mentioned outcome measures.
Results: Of 146 patients, 107 (73.3%) attained complete remission off treatment. Mean interval between first dose rituximab administration and complete remission off treatment was 6.6 ± 3.4months. Complete remission off treatment was sustained for a mean duration of 9.1 ± 8.5 months before relapse. Over a mean follow-up duration of 24.9 ± 17.1 months (median 23, maximum 68 months), 75 of 107 patients (76.5%) who had achieved complete remission after first cycle of rituximab relapsed. A mean total cumulative dose of 3496 ± 2496 mg prednisolone was prescribed over a mean duration of 7.2 ± 4.7 months after first cycle of rituximab. Time taken to achieve remission was significantly longer in pemphigus foliaceus and these patients required significantly higher cumulative dose of prednisolone over a longer duration after rituximab. No deaths and long-term complications were recorded.
Limitations: Only clinical parameters were assessed. Immunological parameters including B-cell counts and enzyme-linked immunosorbent assay for anti-desmoglein antibody titers were not carried out.
Conclusion: This study reinforces the beneficial role of rituximab in pemphigus. Pemphigus foliaceus patients required a higher total cumulative dose of prednisolone over a longer time to achieve remission and the remission lasted longer than that in pemphigus vulgaris.
Introduction
Pemphigus is a potentially life-threatening autoimmune disorder characterized by formation of antibodies against intercellular adhesion molecules in the epidermis resulting in blistering on skin and mucosae. High-dose corticosteroids in conjunction with conventional immunosuppressants have been the cornerstone of treatment for pemphigus. Rituximab, an anti-CD20 chimeric monoclonal antibody, was found to have significant efficacy in corticosteroid dependent and refractory pemphigus.[1] Recently, Joly et al. have recommended the use of rituximab as first-line treatment in treatment-naïve pemphigus patients.[2] Dose and frequency of rituximab administration and outcome measures vary in different studies resulting in heterogeneity of the data generated. Apart from the two meta-analyses,[3],[4] a retrospective analysis by Heelan et al.,[5] a systematic review by Amber and Hertl[6] and another prospective study by Joly et al.,[2] other studies are a series of much smaller number of patients. Here, we assessed long-term clinical response in 146 pemphigus patients who received rituximab as per rheumatoid arthritis protocol.
The primary objective of this study was to assess the effectiveness of biosimilar rituximab in a cohort of 146 pemphigus patients in terms of following primary outcome measures: proportion of patients achieving complete remission off treatment, time to achieve complete remission off treatment, proportion of patients who relapsed after achieving complete remission off treatment, time taken to relapse, total cumulative dose of corticosteroids administered after rituximab and duration of administration of corticosteroids after rituximab.
In addition, we tried to find correlation between some clinicodemographic variables such as age, gender, total duration of illness before rituximab administration and pemphigus disease type (pemphigus vulgaris/ pemphigus foliaceus) with the above-mentioned outcome measures.
Methods
This is a review of the pemphigus patients registered in the immunobullous disease clinic at the Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. Patients were administered biosimilar rituximab manufactured by a single pharmaceutical company from January 2011 to March 2017, and the study was not sponsored.[7] For the patients who had received rituximab before November 2013 and got registered to the clinic later on, information was accessed from their personal medical records. Those who were not following up at the time of extraction of data were contacted on telephone and records were updated on their visit to the clinic. Pemphigus had been diagnosed based on the clinical features, presence of acantholytic cells on Tzanck smear (a screening test), and confirmation of characteristic histopathology (subcorneal clefting in pemphigus foliaceus and suprabasal clefting in pemphigus vulgaris) and direct immunofluorescence findings (deposition of immunoglobulin G and/or complement C3 in intercellular space in fish-net pattern). The definitions for remission on minimal treatment, complete remission off treatment and relapse were the same as described by Murrell et al. in their joint consensus statement.[8]
All patients with diagnosed pemphigus who received two doses of rituximab, 1g 14 days apart, within the study period specified above (n = 146) were included in the study. Eleven patients who did not have complete follow-up data were excluded [Figure - 1]. Rituximab was used for severe (involvement of skin surface area >10%) or recalcitrant disease (minimal response after adequate treatment for >3 months). Adequate treatment was defined as 2.5 mg/kg/day of azathioprine or cyclophosphamide and 0.5–1mg/kg/day of prednisolone. Patients who had received concurrent, preceding or subsequent intravenous immunoglobulin (n = 13) or intravenous cyclophosphamide pulse (n = 10); or oral cyclophosphamide, azathioprine or mycophenolate mofetil (n = 17) for more than 2 weeks after rituximab were excluded [to avoid confounding bias generated from the use of multiple immunosuppressives, thus maintaining homogeneity of data.] Flow of patients in the study is summarized in [Figure - 1]. Patients who had received 500 mg rituximab (instead of 1000 mg, n = 11) on days 1 and 14 were not considered for analysis. Injection hydrocortisone 100 mg, paracetamol 300 mg and pheniramine 22.75 mg was routinely administered 30 min before every rituximab infusion as premedication.
 |
| Figure 1: A flowchart depicting the methodology of the study |
As per the clinic protocol, prednisolone was started at a dose of 40 mg/day with rituximab. After 2 weeks, gradual tapering of prednisolone dose was done by 10 mg every alternate week. After reaching a dose of 10mg/day, prednisolone was tapered by 2.5 mg every 2 weeks. If the new lesions kept appearing and/or the existing lesions had not started healing, tapering was not started and the same dose was continued until next visit. If oral cyclophosphamide or azathioprine was being administered before rituximab, the same was stopped within 15 days of second dose of first cycle rituximab. Relapse was defined as per joint consensus statement.[8] All relapses were initially treated with prednisolone at 20 mg/day. If no response was observed within 2 weeks, dose was subsequently increased to 40 mg/day and azathioprine or cyclophosphamide was added in dose of 2mg/kg/day. If still uncontrolled after 2 months of relapse, patients were offered second cycle of rituximab.
Statistical analysis
SAS version 9.4 was used for the analysis. Descriptive analyses were conducted to determine the distributions (means and proportions for continuous and categorical variables of interest, respectively, with corresponding standard deviations for means).
Next a group of inferential statistical tests were conducted to determine the influence of one variable on the other to address the objectives of the study. Based on the types of variables in consideration, the nature of the tests varied appropriately. Owing to the lack of meeting specific target distributional assumptions, at times different types of tests were also applied to check the sensitivity of the measures of associations and the inferential statistics.
While determining the relationship between two continuous variables (like days of interval between doses and days taken for remission etc.), correlation, simple linear regression and analysis of maximum likelihood parameter estimates using GENMOD procedure (separately for normal and Poisson distributional assumptions for rates) were used.
On the contrary, to determine the association between a categorical independent and a continuous dependent variable, analysis of maximum likelihood parameter estimates using GENMOD procedure and generalized linear mixed regression methods were used.
For categorical dependent variables logistic regression modeling was done. In case of having appropriate potential assumptions, Cox proportional hazard regression was conducted.
For all kinds of regressions, simple unadjusted modeling was followed by adjustments for the potential confounders (identified based on literature review). For all analysis to test statistical significance, α was assumed to be 0.05.
Results
Patients
A total of 146 patients were studied [pemphigus vulgaris = 130 (mucocutaneous 120, pure cutaneous and mucosal, 5 each), pemphigus foliaceus = 16]. Mean (±standard deviation) age of patients was 42.9 ± 14.1 years. There were 65 males (44.5%) and 81 females (55.5%). Onset of disease was from oral mucosa and skin in 52 and 94 patients, respectively. Mean duration of disease before rituximab administration was 21.1 ± 29.8 months (range 1–180 months). The patients were followed up for a mean duration of 24.9 ± 17.1 months (range 0.5–68 months, median 23 months) after administration of first cycle rituximab [Table - 1].
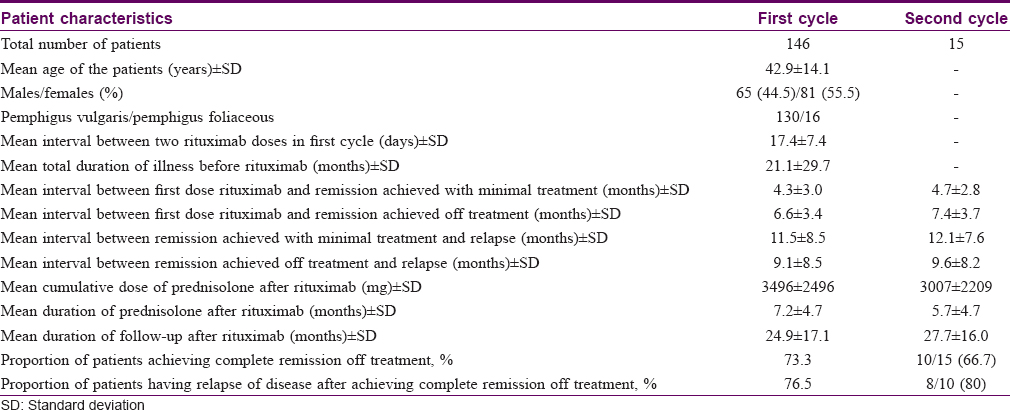
Outcome parameters after first cycle rituximab
Treatment
First cycle of rituximab consisted of two doses, 1 g each, administered at a mean interval of 17.4 ± 7.4 days. Logistic difficulty of following up in time led to delay between two doses of rituximab in some patients, despite being planned 14 days apart.
Remission
Of 146 patients, 107 (73.3%) attained complete remission off therapy. Mean interval between first dose rituximab administration and complete remission achieved on minimal treatment (10 mg/day prednisolone) was 4.3 ± 3.0 months, and that between administration of first dose rituximab and complete remission achieved off treatment was 6.6 ± 3.4 months.
Relapse
The mean follow-up duration was 24.9 ± 17.1 months (median 23, maximum 68 months). Seventy-five of 107 (76.5%) patients who had achieved complete remission after first cycle of rituximab relapsed. The mean interval between achievement of remission on minimal treatment and relapse was 11.5 ± 8.5 months. Complete remission off treatment was sustained for a mean duration of 9.1 ± 8.5 months before relapse. A mean total cumulative dose of 3496 ± 2496 mg prednisolone was required over a mean duration of 7.2 ± 4.7 months after first cycle of rituximab [Table - 1].
Outcome parameters after second cycle rituximab
Second cycle of rituximab was administered to 15 patients. The mean interval between first and second cycle of rituximab for these 15 patients was 18 ± 10.3 months. The mean follow-up period after second cycle of rituximab was 27.7 ± 16.0 months (maximum: 54 months, minimum: 4 months, median: 22 months). Of these, six patients had not attained remission after first cycle rituximab, while the other nine patients had relapsed after attaining remission after first cycle. Notably, two patients (one of mucocutaneous pemphigus vulgaris and another of pemphigus foliaceus) who had not attained remission after the first cycle did not attain remission even after the second cycle of rituximab. The outcome measures after second cycle is summarized in [Table - 1].
Correlation between clinicodemographic parameters and outcome parameters
In addition to assessment of outcome measures described earlier, we tried to find if any correlation existed between selected clinicodemographic parameters and outcome parameters as detailed earlier. No correlation was observed between gender and any of the outcome parameters.
Mean age was not found to correlate significantly with proportion of patients achieving remission or having relapse (P = 0.5 and 0.4, respectively), time to achieve remission and duration of remission (P = 0.8 and 0.5, respectively). A significant negative association was seen between age and cumulative dose and duration of prednisolone required after rituximab administration (P < 0.001, 95% confidence interval:−14.8224 to −13.4626; P = 0.008, 95% confidence interval:−0.0734 to −0.0107, respectively), assuming Poisson's distribution. In both bivariate and multivariable logistic regression models, a higher age was negatively associated with odds of requirement of second cycle of rituximab (P = 0.01). None of the outcome parameters were found to correlate with total disease duration.
There was no significant association between type of pemphigus (pemphigus vulgaris or foliaceus) and proportion of patients achieving remission and having subsequent relapse. Time taken to achieve remission was significantly longer in pemphigus foliaceus, after applying generalized multiple mixed linear regression model (P = 0.036, 95% confidence interval: 0.1504–4.6727). Pemphigus foliaceus patients required significantly more cumulative dose of prednisolone over a longer period after rituximab (P = 0.008 [95% confidence interval: 464.19–3107.71], P < 0.001 [95% confidence interval: 1.8246–6.7209], generalized mixed linear regression). Time taken to relapse was found to have a significant correlation with type of disease, and pemphigus foliaceus lesions took significantly more time to relapse (t-test, P = 0.004).
Complications/adverse events
Of 146 patients, five developed pruritic urticarial rash during first infusion. The infusion had to be briefly discontinued and was restarted at a slower rate after administration of intravenous antihistamine and dexamethasone. All these patients had uneventful subsequent infusions. One patient developed angioedema and bronchospasm during first infusion. Two patients developed pyo-pneumothorax and sepsis each, before second infusion and were treated with appropriate antibiotics, while two patients had severe infusion reactions requiring discontinuation of rituximab during second dose administration. These seven patients were not included in the effectiveness analysis, since they received only one dose of rituximab. No other long-term complications were noted. No deaths were observed during hospital admission in the 146 patients studied, even on review of in-patient records.
Discussion
Rituximab decreases the production of antidesmoglein antibodies without significantly affecting the antimicrobial antibodies.[9] Efficacy of rituximab in severe pemphigus was first reported in 2006 and 2007 by Ahmed et al. and Joly et al., respectively.[1],[10] Rituximab has since been increasingly used by both rheumatoid arthritis protocol and lymphoma protocol to treat pemphigus, both treatment-naïve and refractory, owing to its safety profile and efficacy.[11],[12],[13]
Joly et al. recently reported a complete remission off treatment rate of 89% in patients treated with rituximab and short-term prednisone combination, whereas it was 34% in the prednisone alone group. Cumulative dose of prednisone was one-third in rituximab group (6143.1 mg) as compared to prednisone alone (17973.6 mg) group.[2] In a series of 25 patients treated with rheumatoid arthritis protocol or modifications thereof by Sharma et al., mean total cumulative dose of prednisolone required was 3535.4 mg.[14] The required mean total cumulative dose of prednisolone after first cycle in the present study was comparable at 3496 mg.
The observation that the mean time taken to achieve complete remission off treatment after first cycle rituximab (6.6 months) was lesser than the mean total duration for which prednisolone was required (7.2 months) can be explained because, for assessment of total duration of prednisolone required, all patients including those who did not achieve remission and thus required longer period of treatment with prednisolone, were analyzed.
Ahmed et al. in their meta-analysis observed complete remission on and off therapy in 46.8 and 42% of the patients, respectively. Relapse occurred after 15.7 months of rituximab in 67% patients, and 85.6% patients received additional cycles of rituximab.[4] In another meta-analysis, Wang et al. found complete remission in 76% patients after 5.8 months of first cycle of rituximab.[3] Remission was sustained for a mean duration of 14.5 months and relapse was seen in 40% of patients. Heelan et al., in a large single-center study of 92 refractory/severe pemphigus patients (84 pemphigus vulgaris, 8 pemphigus foliaceus) treated with modified fixed-dose rheumatoid arthritis protocol up to seven cycles, reported improvement in all patients with complete remission in 98% (70 and 28% off and on adjuvants, respectively).[5] Median time to relapse after rituximab was 15 months.
Increased duration of disease before rituximab has been known to correlate with failure to achieve complete remission and significantly increased relapse rate.[6],[12] We, however, could not find a correlation between longer duration of disease before rituximab with remission or relapse rates. Unlike Heelan et al., in our study, patients having pemphigus foliaceus took significantly longer time to achieve remission, and required significantly longer duration and higher cumulative dose of corticosteroids post-rituximab.[5] Pemphigus foliaceus patients also attained a significantly longer duration of remission. As observed by Amber and Hertl, there was no correlation between age and type of disease with probability of remission.[6]
We have analyzed data of patients receiving only corticosteroids post rituximab. Though complete remission off treatment was the primary outcome measure of this study, 33 patients had significant improvement and achieved partial remission. The proportion of patients achieving complete remission off treatment after employing high-dose corticosteroids and conventional immunosuppressants has been observed to be around 30–50% and the remission does not last long.[9] Though rituximab is costly perse, it was shown in a previous study to reduce the overall cost expenses significantly (by 30.3%) after 6 months because of a much reduced need for intravenous immunoglobulins, concomitant immunosuppression, specialist referrals, day-care and in-patient admissions.[15]
Limitations
Only clinical parameters were assessed in this study. The results could have been more representative if immunological parameters including periodic B-cell-counts and anti-desmoglein enzyme-linked immunosorbent assay titers were carried out. Unfortunately, resource constraints precluded such efforts. Owing to the retrospective record-based nature of the study, there were some missing values due to the incomplete follow-up information. But the range of the percentage of the missing values were not high (4–6%) and as they were missing irrespective of any potential association with both the exposure and outcome simultaneously, they were dealt as missing at random and thus we did a complete subject analysis for each of the sets of the variables. Lack of an adequate number of observations resulted in lack of power in some of our inferential sub-analyses, which have not been described. Some potential sparse data bias and residual confounding should also be borne in mind despite our sincere efforts to minimize them through several robust analytical processes.
To conclude, present study reinforces the effectiveness of rituximab in the hitherto largest single-center review following a uniform treatment protocol and also provides an insight into the effect that the disease subtypes might have on the treatment outcomes. The characteristics of patients not responding to rituximab needs to be studied further.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med 2006;355:1772-9.
[Google Scholar]
|
| 2. |
Joly P, Maho-Vaillant M, Prost-Squarcioni C, Hebert V, Houivet E, Calbo S, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): A prospective, multicentre, parallel-group, open-label randomised trial. Lancet 2017;389:2031-40.
[Google Scholar]
|
| 3. |
Wang HH, Liu CW, Li YC, Huang YC. Efficacy of rituximab for pemphigus: A systematic review and meta-analysis of different regimens. Acta DermVenereol 2015;95:928-32.
[Google Scholar]
|
| 4. |
Ahmed AR, Shetty S. A comprehensive analysis of treatment outcomes in patients with pemphigus vulgaris treated with rituximab. Autoimmun Rev 2015;14:323-31.
[Google Scholar]
|
| 5. |
Heelan K, Al-Mohammedi F, Smith MJ, Knowles S, Lansang P, Walsh S, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol 2014;150:703-8.
[Google Scholar]
|
| 6. |
Amber KT, Hertl M. An assessment of treatment history and its association with clinical outcomes and relapse in 155 pemphigus patients with response to a single cycle of rituximab. J Eur Acad Dermatol Venereol 2015;29:777-82.
[Google Scholar]
|
| 7. |
Declerck P, Danesi R, Petersel D, Jacobs I. The language of biosimilars: Clarification, definitions, and regulatory aspects. Drugs 2017;77:671-7.
[Google Scholar]
|
| 8. |
Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol 2008;58:1043-6.
[Google Scholar]
|
| 9. |
Schmidt E. Rituximab as first-line treatment of pemphigus. Lancet 2017;389:1956-8.
[Google Scholar]
|
| 10. |
Joly P, Mouquet H, Roujeau JC, D'Incan M, Gilbert D, Jacquot S, et al. Asingle cycle of rituximab for the treatment of severe pemphigus. N Engl J Med 2007;357:545-52.
[Google Scholar]
|
| 11. |
De A, Ansari A, Sharma N, Sarda A. Shifting focus in the therapeutics of immunobullous disease. Indian J Dermatol 2017;62:282-90.
[Google Scholar]
|
| 12. |
Anandan V, Jameela WA, Sowmiya R, Kumar MM, Lavanya P. Rituximab: A magic bullet for pemphigus. J Clin Diagn Res 2017;11:WC01-6.
[Google Scholar]
|
| 13. |
Bhattacharjee R, De D, Handa S, Minz RW, Saikia B, Joshi N, et al. Assessment of the effects of rituximab monotherapy on different subsets of circulating T-regulatory cells and clinical disease severity in severe pemphigus vulgaris. Dermatology 2016;232:572-7.
[Google Scholar]
|
| 14. |
Sharma VK, Bhari N, Gupta S, Sahni K, Khanna N, Ramam M, et al. Clinical efficacy of rituximab in the treatment of pemphigus: A retrospective study. Indian J Dermatol Venereol Leprol 2016;82:389-94.
[Google Scholar]
|
| 15. |
Heelan K, Hassan S, Bannon G, Knowles S, Walsh S, Shear NH, et al. Cost and resource use of pemphigus and pemphigoid disorders pre- and post-rituximab. J Cutan Med Surg 2015;19:274-82.
[Google Scholar]
|
Fulltext Views
6,821
PDF downloads
3,031





