Translate this page into:
Efficacy and safety of combinations of H1 antihistamines in the treatment of urticaria: A scoping review
Corresponding author: Dr. Fushan Tang, Department of Clinical Pharmacy, School of Pharmacy, Zunyi Medical University, Zunyi, 563006, China. fstang@vip.163.com
-
Received: ,
Accepted: ,
How to cite this article: Luo M, Shen K, Dong X, Zhang W, Tang F. Efficacy and safety of combinations of H1 antihistamines in the treatment of urticaria: A scoping review. Indian J Dermatol Venereol Leprol. 2025;91:49-58. doi: 10.25259/IJDVL_1218_2023
Abstract
The efficacy and safety of combining H1 antihistamines (AHs) for treating urticaria are currently unclear. This scoping review aims to provide a comprehensive overview of the evidence regarding the efficacy and safety of H1 AH combinations in the management of urticaria up to May 2023. The search encompassed databases such as PubMed, Web of Science, the Cochrane Central Register of Controlled Trials, and the China Biological Medicine Database. The inclusion criteria comprised randomised controlled trials (RCTs), non-randomised trials (NRTs), case reports, and case series focusing on urticaria treatment. Initially screening 12,887 studies, this review ultimately selected 109 studies involving 11,435 patients. These studies documented 43 different combination treatments across 11 types of urticaria. In comparison to monotherapy, combination therapy exhibited superior efficacy in 94 studies that reported treatment efficacy. Regarding adverse drug reactions (ADRs), 67 studies disclosed ADR incidences, with combination therapy showing lower ADR rates in 32 studies. Additionally, 7 studies reported similar ADR rates between combination therapy and monotherapy with AHs. Common ADRs included symptoms such as drowsiness, nausea, fatigue, dry mouth, dizziness, and headache, while less frequent side effects encompassed hypotension, otitis media, polyuria, rhinorrhoea, abnormal liver function, and rash. ADR rates ranged from 0% to 21% in the treatment group, and from 0.5% to 75% in the control group. Importantly, patients generally tolerated these ADRs well, with symptoms resolving upon discontinuation of treatment. The study’s findings suggest that combining AHs leads to enhanced efficacy and reduced safety risks compared to monotherapy in the context of urticaria treatment. These results advocate for considering combination therapy as a viable option in clinical practice, especially for chronic urticaria cases. Nonetheless, caution is advised, and close monitoring for potential ADRs is crucial during treatment.
Keywords
adverse drug reactions
combination drug therapy
efficacy
H1 antihistamines
urticaria
Introduction
Urticaria is a common and diverse inflammatory skin condition characterised by the activation and release of histamine and other mediators from skin mast cells, resulting in transient wheals, angioedema, or both.1–5 It can be triggered spontaneously or by various factors, affecting individuals of all ages, with a lifetime prevalence of up to 20% worldwide.5,6 Urticaria can be classified as acute (lasting up to 6 weeks) or chronic (lasting more than 6 weeks) and as inducible (with identifiable triggers) or spontaneous (without specific triggers). While most cases are spontaneous, chronic urticaria is more prevalent in adult women, significantly impacting their quality of life.4,7,8
The treatment of urticaria typically involves first-line therapy with second-generation H1 antihistamines (sgAHs) and alternative options such as omalizumab or cyclosporine-A for patients not responding adequately to sgAHs. Long-term use of corticosteroids is generally not recommended.9 In recent years, emerging treatments have been studied to improve the management of urticaria. For example, up-dosing sgAHs up to four times the standard dose has shown better itch relief in patients with chronic urticaria refractory to conventional doses, as recommended by current guidelines.10 In addition, research on biological agents and small molecule drugs including immunoglobulins, TNF-α inhibitors, IL-1 inhibitors and anti-NK-1R agents have provided promising results.10,11 However, these therapies may carry more serious adverse drug reactions (ADRs), particularly in special populations such as pregnant women, children and the elderly, limiting their use as first-line treatments. Therefore, combining these agents with AHs as a second-line approach has been proposed.12
While studies have suggested that combination therapy with AHs may not offer superior efficacy compared to increasing the dosage of a single AH, this conclusion needs validation through large-scale randomised, controlled and blinded trials.11 Moreover, increasing the dosage of a single AH may lead to increased ADRs, whereas combining AHs with different mechanisms of action could potentially enhance efficacy and reduce safety concerns in patients. Unfortunately, there is a lack of comprehensive research in this area. To bridge this knowledge gap, our study aims to conduct a scoping review of the available literature investigating the efficacy and safety of combined H1 AH use in the treatment of urticaria.
Methods
Methodological framework
We conducted a scoping review following the methodology proposed by Arksey and O’Malley,13 which consists of five key steps: (a) identifying research questions, (b) searching and identifying relevant studies, (c) selecting studies for inclusion, (d) charting the data and (e) organising, summarising and reporting the results. The primary research question guiding this review was: ‘Is the combination of H1 AHs more effective and has fewer ADRs compared to monotherapy in the treatment of urticaria?’
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients diagnosed with urticaria, (2) intervention involving a combination of H1 AHs, (3) studies reporting outcomes, (4) study designs: Randomised controlled trials (RCT), non-randomised trials (NRT), cohort studies, retrospective cohort studies, case series or case reports and (5) studies published in English or Chinese.
Exclusion criteria: (1) Duplicate studies, (2) studies without relevant outcome measures, (3) studies involving combinations of two or more AHs with other drugs, (4) studies unrelated to the topic of this review (e.g., unrelated drugs or diseases), (5) animal tests or cell experiments and (6) studies with obvious errors in administered doses or missing information.
Search strategy
A systematic search was conducted for studies published up to May 2023 in multiple databases including Embase, PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, China National Knowledge Infrastructure (CNKI) database, Wanfang database, Chinese Scientific Journals Full-Text database (VIP) and China Biological Medicine (CBM) database. Medical subject headings and free-text terms such as ‘ketotifen,’ ‘cyproheptadine,’ ‘loratadine,’ ‘cetirizine,’ ‘fexofenadine,’ ‘desloratadine’ and ‘urticarial’ were used in the search strategy. The detailed search strategy can be found in the Appendix.
Study selection and data extraction
All identified studies were imported into Endnote X9, a reference management software, for organisation and management. Duplicate studies were then removed to ensure that only unique studies were included in the review. Two reviewers then independently screened the titles and abstracts of the identified studies for eligibility. Discrepancies were resolved through discussion or involving a third reviewer. Data extraction forms were created to record relevant information including the first author, type of disease, year, gender, age, treatment regimen and duration, treatment outcomes and ADRs.
Synthesis and presentation of results
The results were synthesised and presented using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines, as outlined in the Appendix. The characteristics of the included studies were summarised in tables. In cases where the rates of treatment effectiveness and ADRs were not reported, a custom formula based on the methods reported by Li et al.14 was used to calculate these rates.
Statistical analysis
Statistical analysis was conducted using SPSS 18.0 with t-tests for normally distributed data expressed as mean ± standard deviation and non-parametric tests for skewed distribution data expressed as median M (P25, P75). P < 0.05 was considered statistically significant.
Results
Study selection and the baseline characteristics
A comprehensive search was conducted, resulting in identification of a total of 12,887 studies. After removing duplicates, a total of 109 studies were deemed eligible for inclusion in this review. These included 77 RCTs,14–90 22 NRTs,91–112 7 case reports,113–119 and 3 case series.120–122 The screening process is illustrated in Figure 1.
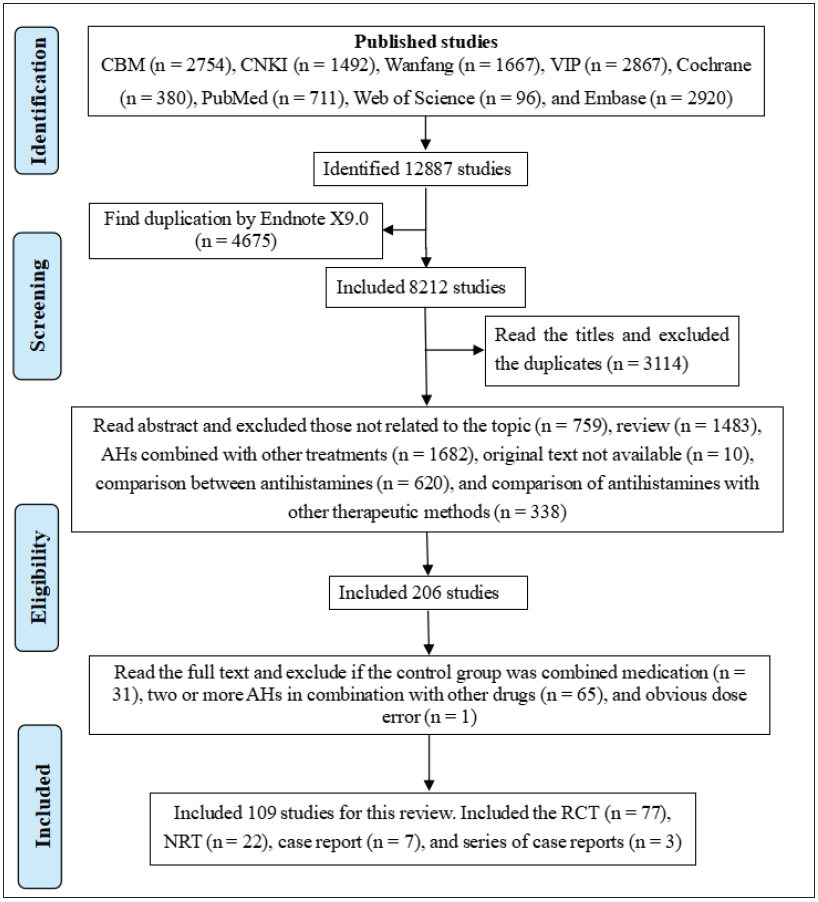
- PRISMA flowchart of study selection and inclusion process (AHs: H1 antihistamines, RCT: randomised controlled trials, NRT: non-randomised trials, CNKI: China national knowledge infrastructure, VIP: Chinese scientific journals full-text database, CBM: China biological medicine).
Among the included studies, 103 were conducted in China, while 2 studies were from Germany,33,91 1 from the United States,115 1 from Portugal114 and 2 studies did not report nationality.113,116 Collectively, the 109 studies involved a total of 11,435 patients with urticaria. Of these, the average age of onset for patients was reported in 101 studies, ranging from 1 to 85 years old. This information provides valuable insights into the demographics of the included patient population.
A total of 109 studies were conducted to address 11 different types of urticaria which included the following: urticaria (n = 7),21,22,94,111,113,116,119 chronic idiopathic urticaria (n = 2),27,122 chronic refractory urticaria (n = 4),14,77,84,89 chronic spontaneous urticaria (n = 4),33,56,105,110 refractory urticaria (n = 10),45,49,61,64,75,76,101,104,106,108 pruritus urticaria (n = 1),91 acute urticaria (n = 1),118 intractable urticaria (n = 1),117 urticaria (angioedema) (n = 1),115 urticaria (mast cell proliferation) (n = 1)114 and chronic urticaria (n = 77).15-20,23-26,28-32,34-44,46-48,50-55,57-60,62,63,65-74,78-83,85-88,90,92,93,95-100,102,103,107,109,112,120,122
Within these studies, a total of 43 combination therapy schemes involving AHs were reported. The breakdown of the combinations is as follows: 2515,16,20,23,24,28,29,32,34,35,38,39,43,48,50,52,53,5 5,58,92,95,99,113,120,122 reported First-generation AHs (fgAHs) and Second-generation AHs (sgAHs) combination, 1717,19,25,27,30,31,33,37,40,41,44,60,62,87,114,115,121 reported fgAHs and Third-generation AHs (tgAHs) combination, 1714,22,47,57,59,63,67,69,72,74,77,80,82,89,93,105,112 reported sgAHs and sgAHs combination, 2418,21,26,51,56,65,73,83-85,88,90,91,96-98,100,103,107,109-111,115,119 reported sgAHs and tgAHs combination, 2336,42,45,46,49,54,61,64,66,68,71,75,76,78,79,81,86,94,101,102,104,106,108 reported tgAHs and tgAHs combination and 3116-118 reported fgAHs and sgAHs and tgAHs combination. Of all the studies, 94 reported the effective rate (rate of efficacy) of treatment and 67 reported ADR rates. For further details on the results of the AHs combinations, please refer to Tables 1 and 2.
| Intergenerational drug combination | Combination of AHs | Number of articles | Number of articles reporting efficacy | Number of effective articles (I > C) | Number of articles reporting the incidence of ADR | Number of articles on incidence of ADR (I < C) |
|---|---|---|---|---|---|---|
| fgAHs + sgAHs (n = 9) |
Carritin + Cyproheptadine (n = 1); Loratadine + Ketotifen (n = 1); Cyproheptadine + Loratadine (n = 3); Mizolastine + Cyproheptadine (n = 9); Mizolastine + Ketotifen (n = 2); Mizolastine + Chlorphenamine Maleate (n = 1); Cetirizine + Promethazine (n = 3); Ebastine + Cyproheptadine + Dosepin (n = 1); Ebastine + Cyproheptadine (n = 1) |
22 | 22 | 21 | 18 | 5 |
| fgAHs + tgAHs (n = 8) |
Levocetirizine + Hydroxy azine (n = 1); Desloratadine + Ketotifen (n = 1); Fexofenadine + Ketotifen (n = 1); Desloratadine Citrate + Ketotifen (n = 3); Desloratadine Citrate + Cyproheptadine (n = 1); Desloratadine Citrate + Chlorphenamine Maleate (n = 1); Levocetirizine + Ketotifen (n = 5); Fexofenadine + Chlorphenamine Maleate (n = 1) |
14 | 13 | 13 | 5 | 4 |
| sgAHs + sgAHs (n = 7) |
Avastin + Loratadine (n = 4); Olotadine + Cetirizine (n = 1); Loratadine + Clomastine (n = 2); Ebastine + Lupatadine (n = 1); Loratadine + Cetirizine (n = 8); Statin + Loratadine (n = 1); Imestine + loratadine (n = 1) |
17 | 16 | 16 | 10 | 4 |
| sgAHs + tgAHs (n = 11) |
Desloratadine + Ebastine (n = 2); Levocetirizine + Ebastine (n = 6); Betastin + Levocetirizine (n = 1); Desloratadine + Loratadine (n = 2); Desloratadine + Mizolastine (n = 2); Cetirizine + Desloratadine (n = 3); Desloratadine Citrate + Cetirizine (n = 2); Levocetirizine + Loratadine (n = 2); Fexofenadine + Loratadine (n = 1); Epistine + Fexofenadine (n = 1); Levocetirizine + Fexofenadine + Azolastine (n = 1) |
23 | 21 | 21 | 16 | 10 |
| tgAHs + tgAHs (n = 4) |
Desloratadine Citrate + Fexofenadine (n = 10); Levocetirizine + Desloratadine Citrate (n = 3); Levocetirizine + Fexofenadine (n = 2); Levocetirizine + Desloratadine (n = 8) |
23 | 19 | 19 | 16 | 9 |
AHs: H1 antihistamines, ADR: adverse drug reaction, I: intervention group, C: control group, fgAHs: first-generation AHs, sgAHs: second-generation AHs, tgAHs: third-generation AHs, I<C: the effective rate of intervention group was higher than that of control group, I<C: adverse drug reactions in intervention group were less than those in control group.
| Number of articles | Combination of AHs | Intergenerational drug combination | Report the outcomes of treatment | Improved and effective | Report ADRs | No serious ADRs were reported |
|---|---|---|---|---|---|---|
| 10 | Fexofenadine + Cetirizine + Ketotifen (n = 1); Lupatadine + Desloratadine Citrate (n = 1); Mizolastine + Ketotifen + Levocetirizine (n = 1); Carritin + Chlorphenamine Maleate + Levocetirizine (n = 1); Hydroxy azine + Fexofenadine (n = 1); Levocetirizine + Ketotifen (n = 2); Ketotifen + Cetirizine (n = 1); Cetirizine + Cyproheptadine (n = 1); Loratadine + Chlorphenamine Maleate (n = 1) |
sgAHs + tgAHs (n = 1); sgAHs + sgAHs (n = 0); fgAHs + tgAHs (n = 3); fgAHs + sgAHs (n = 3); fgAHs + sgAHs + tgAHs (n = 3); |
10 | 6 | 2 | 2 |
AHs: H1 antihistamines, ADR: adverse drug reaction, fgAHs: First-generation AHs, sgAHs: second-generation AHs, tgAHs: third-generation AHs
In the studies analysed, a range of different approaches to medication administration was observed. In 26 studies, the frequency of administration was reduced in the treatment group compared to the control group.15,16,20,24,27,28,30,31,35,37-41,43,48,53,82,87,92,95,99,105,114,120,122 However, in two studies,33,107 both groups had an increase in the dose, reaching up to four times the conventional dosage. In two other studies,83,109 only the dose of the treatment group was increased which was twice as much as the conventional dose. Interestingly, one study71 increased the dose only in the control group, resulting in a dosage that was twice that of the conventional dose, but this led to an increase in the incidence of ADRs. The treatment duration varied among these studies with the longest course of treatment lasting for 1 year,66 while the shortest duration being only 5 days.33,113 The course of treatment was not reported in 15 studies.37,38,53,61,75,76,91,103,104,107,114-116,118,119
Clinical outcome of the study (efficacy and safety)
Of the total number of studies analysed, 94 of them reported the effective rate of the treatment under consideration. The effectiveness of the treatment varied among these studies with the lowest recorded effective rate being 60.4%14 and the highest effective rate reaching 100%79 in the combined group. In contrast, for the control group, the lowest effective rate observed was 37.3%,14 while the highest effective rate stood at 99.5%.92 Among the 94 studies, only 1 study showed a slightly lower efficacy with combination therapy (98% vs 99.5%),92 but the difference was not statistically significant (P > 0.05). Several other outcomes were reported in these studies. Some studies indicated a reduction of inflammatory factors (n = 5),49,63,71,76,106 improvement in quality of life (n = 1),33 decrease in recurrence rate (n = 1),21 amelioration of symptoms (n = 4),114–116,118 presence of recurrent symptoms (n = 1),119 relief from itching (n = 1),91 no change in symptoms (n = 1)113 and one study reported the treatment as ineffective (n = 1).117
A total of 67 studies provided information on the incidence of ADR. Among these studies, combination therapy demonstrated a lower ADR incidence compared to monotherapy in 32 studies.17,22,23,27,34,40,41,45,46,48,51-53,59,66,68-71,74,76,79,84,85,88,90,100,103,104,106,110,111 In seven studies,16,18,20,26,28,58,67 the ADR incidence was found to be equal between the two treatment approaches. Thirty-four studies did not provide data on the incidence of ADR. In addition, six studies31,35,38,44,87,91 mentioned the occurrence of ADRs but did not report the specific incidence rates. Only two studies15,93 reported the incidence of ADR rates, but they provided information for only one of the treatment groups. The general ADRs reported included drowsiness, nausea, fatigue, dry mouth, dizziness and headache. Slightly more serious ADRs included hypotension, otitis media, polyuria, rhinorrhoea, abnormal liver function, rash, loss of appetite and pain in other parts of the body. These ADRs were observed in different combinations of fgAHs and sgAHs, fgAHs and tgAHs, sgAHs and sgAHs, sgAHs and tgAHs and tgAHs and tgAHs, respectively. The incidence rates of ADRs ranged from 0% to 21% in the treatment group, while in the control group it varied from 0.5% to 75%. It is worth noting that all ADRs were found to be tolerable by the patients and resolved after discontinuation of the treatment. For more detailed information, please refer to Tables 1–3 in the supplementary materials.
| AHs combination therapy regimen | Results | ||||||
|---|---|---|---|---|---|---|---|
| Mizolastine + Cyproheptadine (n = 9) | Effective rates (n = 9) | P | ADRs (n = 8) | P | |||
| I | C | 0.015 | I | C | 0.721 | ||
| 92.97 ± 5.26b | 79.33 ± 14.15b | 12.50 (9.1, 13.41)a | 10 (9.21, 17.5)a | ||||
| Levocetirizine + Ketotifen (n = 5) | Effective rates (n = 5) | 0.013 | ADRs (n = 2) | 0.895; NA | |||
| I | C | I | C | ||||
| 92.68 ± 4.57b | 80.15 ± 7.53b | NA | NA | ||||
| Loratadine + Cetirizine (n = 8) | Effective rates (n = 7) | 0.003 | ADRs (n = 6) | 0.143 | |||
| I | C | I | C | ||||
| 92.12 ± 7.00b | 77.43 ± 7.68b | 6.12 ± 4.50b | 10.03 ± 2.97b | ||||
| Levocetirizine + Ebastine (n = 6) | Effective rates (n = 6) | 0.003 | ADRs (n = 4) | 0.446 | |||
| I | C | I | C | ||||
| 96.18 ± 3.41b | 81.31 ± 8.92b | 4.95 ± 3.18b | 6.65 ± 2.70b | ||||
| Desloratadine citrate + Fexofenadine (n = 10) | Effective rates (n = 7) | 0 | ADRs (n = 5) | 0.065 | |||
| I | C | I | C | ||||
| 92.71 ± 2.82b | 75.57 ± 3.00b | 6.73 ± 2.39b | 17.93 ± 11.46b | ||||
AHs: H1 antihistamines, ADR: adverse drug reaction, I: intervention group, C: control group, NA: not available/not applicable, a expressed as median M (P25, P75), b Expressed as mean ± standard deviation, n: represents how many articles in which the same H1 antihistamine combination therapy regimen appears.
Common combination therapy schemes
In a total of 99 Randomised controlled trials (RCTs) and Non-randomised trials (NRTs), several combination therapies were identified. These included cyproheptadine and mizolastine in nine studies,16,32,35,43,48,53,55,92,99 levocetirizine and ketotifen in five studies,17,19,25,40,114 loratadine and cetirizine in eight studies,22,47,57,59,63,67,74,93 levocetirizine and ebastine in six studies51,65,70,85,98,100 and loratadine citrate and fexofenadine in ten studies.45,49,61,64,75,76,101,104,106,108 When compared to monotherapy, combination therapy demonstrated superior efficacy with statistical significance (P < 0.05). Furthermore, there was no significant difference observed in the incidence rates of ADR between the two treatment approaches (P > 0.05). Detailed results can be found in Table 3.
Discussion
Specific efficacy and safety of combinations of AHs on urticaria
This scoping review summarises the evidence related to the clinical efficacy and safety of combination therapy with AHs in the treatment of patients with urticaria. Our research questions focused on describing the current literature on H1 AHs combinations for the treatment of urticaria. We found that most studies only looked at the results of AHs combination therapy in general which is usually associated with higher efficacy rates and fewer ADRs than monotherapy. However, the sample size of up to 368 cases in all the studies was not sufficient to draw firm conclusions and therefore our conclusions can only be taken as an inference.
Specifically, combination therapy with AHs is known to be effective in treating urticaria, at least when compared to monotherapy which has better efficacy and fewer ADRs. Although individual studies have reported higher ADRs with combination therapy compared to monotherapy, combination therapy does exert a synergistic effect. It is important to note that urticaria exists in multiple types with the major types being acute urticaria (AU) and chronic urticaria (CU). There are significant differences in the aetiology and treatment of these two types. AU typically subsides within a week of onset, but approximately 40% of patients may progress to develop CU which has a much longer treatment time and may resolve naturally after several years.6 Of the studies we included in our review, only one118 reported on AU as a case report, while the remaining studies focused on CU and other subtypes.
According to the literature we retrieved, most patients with CU choose combination therapy with AHs, while patients with AU tend to receive monotherapy or combined treatment with traditional Chinese medicine (TCM).123–126 While our review focused on the efficacy and safety of combination therapy with H1 AHs for urticaria, it should be noted that further studies are needed to fill the gap in research on the effectiveness and safety of AHs as monotherapy or in combination with TCM for treating acute urticaria (AU). The studies included in our review primarily used second- and third-generation AHs for treating acute urticaria (AU).
It is important to emphasise that the results and conclusions presented in this study apply primarily to patients with CU. The paucity of reported studies and the lack of representativeness of the only study on AU included in our review make it difficult to determine the efficacy of combination therapy with AHs for treating AU. Further studies are needed to address these gaps in knowledge.
In our study, the combinations of sgAHs and tgAHs, sgAHs and sgAHs or tgAHs and tgAHs enhance immune function, reduce cardiac toxicity, improve anxiety and depression and overall quality of life while reducing inflammatory factors. Specifically, the combination of sgAHs and tgAHs effectively blocks histamine receptors without affecting the central nervous system. Studies have reported that the sgAHs ebastine is generally well-tolerated and has minimal adverse cognitive and psychomotor effects.127 Therefore, combining these drugs significantly improves efficacy compared to monotherapy.
fgAHs and sgAHs or fgAHs and tgAHs reduce nocturnal itching, improve sleep quality and increase treatment adherence. However, fgAHs have a strong sedative effect, can cause drowsiness and may prolong the Q-T interval and induce Torsade de Pointes. To mitigate these effects, reducing the dose of fgAHs while concurrently using a sgAHs or tgAHs over the long term can improve efficacy compared to short-term treatment, effectively reducing sleepiness. Long-term therapy can also allow for reduced drug doses, lower medical expenses, minimise sensitivity and maintain immune function. Long-term therapy has been shown to improve treatment outcomes and reduce ADRs compared to short-term treatment.92 It is important to note that the drowsiness effect of combined therapy is stronger in the initial course of treatment and tends to decrease with continued treatment.
Is increasing the dose of a single AH more effective than combination therapy? In a study by Kuang et al.,128 the treatment group received twice the conventional dose of loratadine, while the control group received a combination of loratadine and cetirizine. The results showed that combination therapy was more effective with no significant difference in ADRs between the two groups. Conversely, other studies have suggested that increasing the dose of a single sgAH is preferred over combining different sgAHs.9,129 However, further research is required to confirm the optimal method of drug use through large, well-designed double-blind clinical trials.130 Current evidence suggests that reduced treatment doses can also achieve better efficacy in the case of combination therapy. In our review, 26 studies reported that reducing treatment doses while using combination therapy resulted in improved efficacy compared to the control group with no significant difference in ADRs between the two groups. However, Schulz et al.91 reported lower efficacy with combinations of more than two AHs compared to increasing the dose of a single AH, possibly due to unknown interactions. Future research should aim to confirm the most effective method of drug use.
Discussion on the efficacy and safety risk of combination therapy for urticaria
In our review, all studies except one reported better efficacy with combination therapy than monotherapy. Most ADRs were mild and reversible. Common side effects included drowsiness, dry mouth, dizziness, headache and stomach discomfort which were generally well-tolerated by patients. More serious ADRs such as hypotension, otitis media, abnormal liver function, rash and pain in other parts of the body occurred with the combination of fgAHs and sgAHs, fgAHs and tgAHs, sgAHs and sgAHs and tgAHs and tgAHs, respectively. However, these ADRs returned to normal immediately after discontinuation of the therapy. Combination therapy did not significantly increase the occurrence of ADRs compared to monotherapy. It is important to note that there may be a higher risk of nephrotoxicity with combination therapy and patients receiving combination treatment may also be at a higher risk of treatment failure.131,132 In addition, it is crucial to closely monitor liver function when using sgAHs.14,69,74 Recent guidelines do not recommend the use of fgAHs for the treatment of urticaria due to their significant side effects on the central nervous system which can impair daily activities, especially in special patient groups.133 Therefore, the occurrence of ADRs should not be ignored when using combinations of AHs.
Future Research
There are several areas that require further research: (1) Evaluating the economic benefits of combination therapies, (2) investigating whether lowering drug doses or reducing dosing intervals in combination therapies leads to a lower incidence of ADRs, (3) studying the drug interactions in combination therapies, and (4) assessing drug safety in special populations such as very aged persons or those who are pregnant.
Limitations
Our analysis filled the gap in the literature regarding the combined application of two or more AHs in the treatment of urticaria. However, we did not evaluate publication bias and most of the studies included in our review were conducted in China. Therefore, this conclusion may only be applicable to patients with urticaria in China and caution should be exercised when extrapolating these findings to patients in other countries.
Conclusion
In conclusion, the reviewed studies have consistently demonstrated that combination therapy with two H1 AHs is more effective than AH monotherapy for treating urticaria with the exception of one study. Most ADRs associated with such therapy were mild and reversible and no new safety concerns were observed. The most common combinations were mizolastine and cyproheptadine, levocetirizine and ketotifen, loratadine and cetirizine, levocetirizine and ebastine and desloratadine citrate and fexofenadine.
This review offers valuable guidance to healthcare providers for selecting appropriate combination therapies with AHs for treating urticaria, particularly CU. While combination therapy may be preferred for most CU cases, AU may be treated with AH monotherapy or in conjunction with TCM. However, the use of combination therapy with AHs should always be individualised, considering patient-specific characteristics and closely monitored for response and adverse events.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Epidemiology of urticaria: A representative cross-sectional population survey. Clin Exp Dermatol. 2010;35:869-73.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and risk factors of urticaria with a focus on chronic urticaria in children. Allergy Asthma Immunol Res. 2017;9:212-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The role and relevance of mast cells in urticaria. Immunol Rev. 2018;282:232-47.
- [CrossRef] [PubMed] [Google Scholar]
- The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734-66.
- [CrossRef] [PubMed] [Google Scholar]
- Urticaria: A narrative overview of differential diagnosis. Biomedicines. 2023;11
- [CrossRef] [PubMed] [Google Scholar]
- The challenges of chronic urticaria part 1: Epidemiology, immunopathogenesis, comorbidities, quality of life, and management. World Allergy Organ J. 2021;14:100533.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of urticaria including physical urticaria and angioedema in Korea. Korean J Intern Med. 2019;34:418-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- S3 Guideline Urticaria. Part 2: Treatment of urticaria - German-language adaptation of the international S3 guideline. J Dtsch Dermatol Ges. 2023;21:202-15.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging therapies in chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2019;11:470-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The challenges of chronic urticaria part 2: Pharmacological treatment, chronic inducible urticaria, urticaria in special situations. World Allergy Organ J. 2021;14:100546.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Expert consensus on the use of omalizumab in chronic urticaria in China. World Allergy Organ J. 2021;14:100610.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Scoping studies: towards a methodological framework. International J Social Res Methodology. 2005;8:19-32.
- [Google Scholar]
- A multicenter randomized controlled study of atorvastatin combined with loratadine in the treatment of chronic refractory urticaria. Chin J Dermatol. 2020;53:319-23.
- [Google Scholar]
- Observation on the efficacy of capritan combined with cyproheptadine in the treatment of chronic urticaria. Chin Prim Health Care. 2006;20:75-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical observation of mizolastine combined with cyproheptadine in the treatment of chronic urticaria. Chin J Misdiagn. 2006;6:4165-6.
- [Google Scholar]
- Clinical observation of levocetirizine hydrochloride combined with ketotifen in the treatment of 42 cases of chronic urticaria. J Youjiang Med Univ Ntns. 2008;6:981-2.
- [Google Scholar]
- Clinical observation of fexofenadine hydrochloride combined with loratadine in the treatment of chronic urticaria. Chin J Lepr & Skin Dis. 2008;28:83-4.
- [PubMed] [Google Scholar]
- Clinical analysis of levocetirizine hydrochloride combined with ketotifen in the treatment of 189 cases of chronic urticaria. Chin J Mod Drug Appl. 2009;3:90-1.
- [Google Scholar]
- Clinical observation of mizolastine combined with chlorpheniramine in the treatment of 35 children with chronic urticaria. J Aerosp Med. 2010;21:1865-6.
- [Google Scholar]
- Observation on therapeutic effect of desloratadine combined with ebastine in urticaria. Guide Chin Med. 2010;8:248-9.
- [Google Scholar]
- Observation on the therapeutic effect of loratadine combined with cetirizine in urticaria. J Mil Surg South Chin. 2011;13:457-9.
- [Google Scholar]
- Clinical observation of loratadine in the treatment of chronic urticaria. Seek Med Ask Med. 2012;10:143.
- [Google Scholar]
- Clinical observation of ebastine combined with cyproheptadine decreasing method in the treatment of chronic urticaria. Chin J Postgrad Med. 2012;35:69-70.
- [Google Scholar]
- Levocetirizine combined with ketotifen in the treatment of chronic urticaria. Chin J Prim Med Pharm. 2012;19:2771-2.
- [Google Scholar]
- Clinical efficacy of three methods in treatment of chronic urticaria. J Xinxiang Med Univ. 2013;30:390-2.
- [Google Scholar]
- Clinical curative effect of fexofenadine hydrochloride combined with chlorphenamine maleate in treatment of chronic idiopathic urticaria. Chin J Sch Dr. 2013;27:789-90.
- [Google Scholar]
- Clinical analysis of loratadine long course decreasing therapy in treating chronic urticaria. Chin J Pharm Econ 2013:106-7.
- [Google Scholar]
- Observation on therapeutic effect of loratadine tablets combined with ketotifen fumarate on chronic urticaria. Med Front 2013:184-5.
- [Google Scholar]
- Treatment of chronic urticaria with loratadine citrate combined with chlorpheniramine. Med Info. 2014;27:448-9.
- [Google Scholar]
- Treatment of 58 cases of chronic urticaria with loratadine tablets citrate combined with cyproheptadine hydrochloride tablets. Chin Pharm. 2014;23:121-2.
- [Google Scholar]
- Clinical study of mizolastine combined with cyproheptadine decreasing therapy in the treatment of chronic urticaria. Hebei Med. 2014;20:754-6.
- [Google Scholar]
- Night-time sedating H1 -antihistamine increases daytime somnolence but not treatment efficacy in chronic spontaneous urticaria: A randomized controlled trial. Br J Dermatol. 2014;171:148-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical efficacy of loratadine in patients with chronic urticaria. For All Health 2014:290.
- [Google Scholar]
- Clinical observation of mizolastine combined with cyproheptadine in the treatment of 46 cases of chronic urticaria. J Contemp Clin Med. 2014;27:946.
- [Google Scholar]
- A multicenter clinical observation of levocetirizine combined with desloratadine in the treatment of chronic urticaria in children. Chin J Derm Dermatovenereol. 2015;29:481-3.
- [Google Scholar]
- Clinical observation of fexofenadine combined with ketotifen decreasing therapy in the treatment of chronic urticaria. Chin J Trauma & Dis Med 2015:147-8.
- [PubMed] [Google Scholar]
- Clinical report of mizolastine combined with ketotifen in the treatment of 15 cases of chronic urticaria. Contemp Med. 2015;21:141-2.
- [Google Scholar]
- Clinical observation of ebastine combined with cyproheptadine and doxepin in the treatment of chronic urticaria. Chin Community Dr. 2015;31:69-70.
- [Google Scholar]
- Clinical observation of levocetirizine hydrochloride combined with ketotifen in the treatment of chronic urticaria. Diet Health. 2016;3:57-8.
- [Google Scholar]
- Effct observation of desloratadine citrate combined with ketotifen fumarate in the treatment of chronic urticaria. Chin Mod Med. 2016;23:91-3.
- [Google Scholar]
- A multicenter clinical study of levocetirizine hydrochloride in the treatment of chronic urticaria in children. China Health Care & Nutr. 2016;26:31.
- [PubMed] [Google Scholar]
- Clinical observation of mizolastine combined with cyproheptadine decreasing therapy in treating 94 cases of chronic urticaria. Chin J Derm Dermatovenereol. 2016;30:1095-7.
- [Google Scholar]
- Observation on therapeutic effect of loratadine tablets combined with ketotifen fumarate on chronic urticaria. Drug Eval. 2016;13:281-2.
- [Google Scholar]
- Curative effect of desloratadine citrate and fexofenadine in refractory urticaria. Chin J Biochem & Pharm. 2017;37:249-51.
- [PubMed] [Google Scholar]
- Clinical observation of levocetirizine combined with desloratadine in the treatment of 80 cases of chronic urticaria. Chin J Dermatovenereol. 2017;31:588-90.
- [Google Scholar]
- Curative effect of loratadine and cetirizine for chronic urticaria and the influence on serum IgE. Med Recapitulate. 2017;23:406-9.
- [Google Scholar]
- Mizolastine combined with cyproheptadine decreasing therapy in the treatment of 80 cases of chronic urticaria. Med Front. 2017;7:40-1.
- [Google Scholar]
- Analysis of the clinical effect of loratadine and fexofenadine in the treatment of refractory urticaria. J Clin Med. 2017;4:10438-9.
- [Google Scholar]
- Study on the efficacy of promethazine hydrochloride tablets combined with cetirizine hydrochloride tablets in patients with chronic urticaria. Chin Health Care & Nutr. 2017;27:168.
- [PubMed] [Google Scholar]
- Clinical analysis of levocetirizine hydrochloride combined with ebastin in the treatment of chronic urticaria. Health for everyone 2017:85.
- [Google Scholar]
- Curative effect observation of promethazine hydrochloride tablets and cetirizine hydrochloride tablets in combined treatment of chronic urticaria. Chin Med & Pharm. 2017;7:81-3.
- [PubMed] [Google Scholar]
- Analysis of efficacy and prognosis of cyproheptadine decreasing therapy combined with mizolastine in the treatment of 45 cases of chronic urticaria. J Clin Med Prac. 2017;21:187-8.
- [Google Scholar]
- Effect of the combination of the cetirizine and fexofenadine tablets on serum IgE in patients with chronic urticaria. Med Recapitulate. 2017;23:588-90.
- [Google Scholar]
- Clinical efficacy and safety of mizolastine combined with cyproheptadine in decreasing treatment of chronic urticaria. Mod Diag & Treat. 2017;28:2569-70.
- [PubMed] [Google Scholar]
- Clinical observation of loratadine combined with desloratadine in the treatment of chronic spontaneous urticaria in children. Chin J Dermatol. 2017;50:46-8.
- [Google Scholar]
- Efficacy of cetirizine combined with loratadine in the treatment of chronic urticaria. Med Front. 2018;8:65-6.
- [Google Scholar]
- Clinical analysis of promethazine hydrochloride tablets combined with cetirizine hydrochloride tablets in the treatment of chronic urticaria. Electron J Clin Med Lit. 2018;5:86-7.
- [Google Scholar]
- Therapeutic effect of loratadine combined with cetirizine hydrochloride on chronic urticaria. J North Pharm. 2018;15:121.
- [Google Scholar]
- To observe the effect of levocetirizine and ketotifen on the quality of life in patients with chronic urticaria (idiopathic) Chin Health Care & Nutr. 2018;28:103-4.
- [PubMed] [Google Scholar]
- Clinical observation of loratadine citrate disodium combined with fexofenadine in the treatment of refractory urticaria. Med Forum. 2018;22:1316-7.
- [Google Scholar]
- Effect of desloratadine combined with ketotifen fumarate in the treatment of chronic urticaria. Cardiovas Dis Electron J Integr Trad Chin & West Med. 2018;6:10,2.
- [PubMed] [Google Scholar]
- Therapeutic effect of cetirizine combined with loratadine on chronic urticaria. Hn Med Res. 2019;28:2384-6.
- [Google Scholar]
- Efficacy of loratadine citrate combined with fexofenadine in the treatment of refractory urticaria. Health for Everyone 2019:27.
- [Google Scholar]
- Study on the therapeutic effect of levocetirizine combined with ebastine on chronic urticaria. Chin Community Dr. 2019;35:51-2.
- [Google Scholar]
- Effect of levocetirizine hydrochloride on inflammatory factors in the treatment of chronic urticaria. Med Forum. 2019;23:4657-8.
- [Google Scholar]
- To explore the effect of loratadine combined with cetirizine hydrochloride in the treatment of chronic urticaria. Mod Dig & Interv 2019:2379.
- [PubMed] [Google Scholar]
- Clinical analysis of levocetirizine combined with desloratadine in the treatment of chronic urticaria in children. Mod Diagn & Treat. 2019;30:2964-6.
- [Google Scholar]
- Clinical observation of clemastine fumarate combined with loratadine in the treatment of chronic urticaria in children. Prac Clin J Integr Tradit Chin & West Med. 2019;19:45-7.
- [PubMed] [Google Scholar]
- Clinical efficacy of levocetirizine combined with ebastine in the treatment of chronic urticaria and their effect on serum cytokines. Int J Clinical Exp Med. 2019;12:11675‐83.
- [Google Scholar]
- Effect of levocetirizine combined with desloratadine on serum IgE level and adverse reactions in patients with chronic urticaria. Dermatol & Venereol. 2020;42:531-3.
- [PubMed] [Google Scholar]
- Observation of the effect of olotadine combined with cetirizine in the treatment of chronic urticaria. J Gannan Med Univ. 2020;40:88-6.
- [Google Scholar]
- Efficacy of loratadine citrate combined with cetirizine in the treatment of chronic urticaria and its influence on serum immunoglobulin E level. Dermatol & Venereol. 2020;42:238-40.
- [PubMed] [Google Scholar]
- Clinical observation of loratadine combined with cetirizine drops in the treatment of chronic urticaria. Dermatol & Venereol. 2020;42:240-1.
- [PubMed] [Google Scholar]
- Efficacy of loratadine citrate combined with fexofenadine in the treatment of refractory urticaria. Health for Everyone 2020:102.
- [Google Scholar]
- Clinical observation of desloratadine citrate + fexofenadine in the treatment of patients with refractory urticaria. Heilongjiang Med J. 2020;33:373-5.
- [Google Scholar]
- Efficacy of Avastin combined with loratadine in patients with chronic refractory urticaria. Renowned Dr 2020:173-4.
- [Google Scholar]
- Efficacy and safety of desloratadin citrate + levocetirizine hydrochlorid tablets in the treatment of chronic urticaIia. World J Complex Med. 2020;6:157-9.
- [Google Scholar]
- Clinical efficacy of levocetirizine combined with desloratadine in the treatment of chronic urticaria. Chin J Clin Ration Drug Use. 2020;13:82-3.
- [Google Scholar]
- Clinical study of rupatadine combined with ebastine in treating chronic urticaria. Drugs & Clin. 2020;35:1364-7.
- [PubMed] [Google Scholar]
- Clinical efficacy and safety of levocetirizine combined with desloratadine in the treatment of chronic urticaria in children. Chin J Clin Ration Drug Use. 2021;14:154-6.
- [Google Scholar]
- Clinical observation of setastine hydrochloride combined with loratadine tablets on chronic urticaria and its influence on IgE, WBC and 5-HT levels. J Med Theor & Prac. 2021;34:3205-7.
- [PubMed] [Google Scholar]
- Clinical observation of desloratadine in the treatment of chronic urticaria. Health Manag 2021:90.
- [Google Scholar]
- Efficacy of loratadine combined with levocetirizine in the treatment of chronic refractory urticaria in children and its effects on levels of serum IgE and IFN-γ. Hebei Med. 2021;27:1383-8.
- [Google Scholar]
- Study on the efficacy of ebastin combined with levocetirizine in the treatment of chronic urticaria and its effect on serum IgE level. Database of Chinese Sci-tech Journals (citation edition) Medicine and Health 2021:8-9.
- [Google Scholar]
- Effect of cetirizine combined with desloratadine citrate on chronic urticaria. Chin Health Care & Nutr. 2021;31:186.
- [PubMed] [Google Scholar]
- Efficacy and safety of loratadine citrate combined with ketotifen fumarate in the treatment of chronic urticaria. Clin J Diabetes World. 2021;18:80.
- [Google Scholar]
- To observe the effect of midazolastine combined with desloratadine on chronic urticaria and its effect on sleep quality. World J Sleep Med. 2021;8:2076-8.
- [Google Scholar]
- Efficacy and safety of loratadine combined with avastin in treatment of chronic refractory urticaria. Chin J Sch Dr. 2022;36:289-91.
- [Google Scholar]
- Efficacy and safety analysis of bensulbetastatin combined with levocetirizine hydrochloride in the treatment of chronic urticaria. Contemp Med Forum. 2022;20:129-31.
- [Google Scholar]
- Antipruritic efficacy of a high-dosage antihistamine therapy: Results of a retrospectively analysed case series. Hautarzt. 2009;60:564-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical observation of mizolastine combined with cyproheptadine decreasing therapy in the treatment of chronic urticaria. J Clin Dermatol. 2010;39:193-4.
- [Google Scholar]
- Clinical observation of loratadine combined with cetirizine hydrochloride in the treatment of chronic urticaria. Home Med (Med Trib). 2010;2:663-4.
- [Google Scholar]
- Clinical observation of levocetirizine hydrochloride combined with fexofenadine hydrochloride in the treatment of artificial urticaria. Chin J Dermatovenerol Integr Tradit and West Med. 2013;12:307-8.
- [Google Scholar]
- Clinical observation of mizolastine combined with ketotifen decreasing therapy in the treatment of chronic urticaria. J Clin Dermatol. 2013;42:697-9.
- [Google Scholar]
- Clinical observation of epistin combined with fexofenadine in the treatment of chronic urticaria. Mod Prev Med. 2013;40:1992-3.
- [Google Scholar]
- Analysis of clinical efficacy of different methods in the treatment of chronic urticaria. For All Health. 2014;8:107.
- [Google Scholar]
- Effects of levocetirizine combined with ebastine on laboratory indexes and clinical symptom scores in the treatment of chronic urticaria and clinical curative effect analysis. Hebei Med J. 2016;38:1015-7.
- [Google Scholar]
- Clinical analysis of mizolastine combined with cyproheptadine decreasing therapy in the treatment of chronic urticaria. Guide Chin Med. 2017;15:80.
- [Google Scholar]
- Efficacy of ebastine combined with levocetirizine in the treatment of chronic urticaria and its influence on serum IgE level. Chin J Clin Ration Drug Use. 2018;11:100-1.
- [Google Scholar]
- Efficacy of loratadine citrate disodium combined with fexofenadine in the treatment of refractory urticaria and its influence on immune function. J Shandong First Med Univ & Shandong Acad Med Sci. 2018;39:182-4.
- [PubMed] [Google Scholar]
- Observation on effect of desloratadine citrate tablets combined with levocetirizine hydrochloride tablets in the treatment of chronic urticaria. Doctor. 2019;4:116-7.
- [Google Scholar]
- Clinical analysis of cetirizine hydrochloride combined with desloratadine citrate tablets in the treatment of chronic urticaria. Psychol Mon. 2019;14:185.
- [Google Scholar]
- Efficacy of loratadine combined with fexofenadine in the treatment of refractory urticaria. Health for everyone 2019:245.
- [Google Scholar]
- Effect of avastin combined with loratadine in the treatment of patients with chronic itIiopathic urticaria. J Clin Med Prac. 2020;24:119-21.
- [Google Scholar]
- Clinical efficacy and safety of loratadine combined with fexofenadine in the treatment of refractory urticaria. Database of Chinese Sci-tech Journals (citation edition) Medicine and Health 2020:43-4.
- [Google Scholar]
- Effect of desloratadine tablets combined with cetirizine hydrochloride dispersible tablets on chronic urticaria. World Latest Med Inf. 2020;20:98-9.
- [Google Scholar]
- Clinical observation of desloratadine citrate plus fexofenadine in the treatment of refractory urticaria. Chin Health Horiz 2020:25.
- [Google Scholar]
- Effect of desloratadine tablets combined with cetirizine hydrochloride dispersible tablets on chronic urticaria. World Latest Med Inf. 2020;20:143-4.
- [Google Scholar]
- Clinical efficacy and adverse reactions of desloratadine combined with mizolastine in the treatment of children with chronic spontaneous urticaria. Maternal & Child Health Care of Chin. 2022;37:2826-9.
- [PubMed] [Google Scholar]
- Clinical observation of loratadine combined with levocetirizine in the treatment of urticaria. Chin Sci-tech J Database (full-text version) Med and Health 2022:54-7.
- [Google Scholar]
- Retrospective analysis of Emestine fumarate sustained release capsule combined with loratadine in the treatment of chronic urticaria. Health care Chin. 2022;40:182-4.
- [Google Scholar]
- The acute annular urticaria is now called urticaria multiforme: A case presentation. Annals of Allergy, Asthma & Immunol. 2009;103:A145.
- [PubMed] [Google Scholar]
- Anaphylaxis as presentation of cutaneous mastocytosis: A clinical case. Allergy: Eur J Allergy & Clin Immunol. 2009;64:414.
- [PubMed] [Google Scholar]
- Prevention of kounis syndrome episodes using a combination of ketotifen and non-sedating antihistamines. Annals of Allergy, Asthma & Immunol. 2014;113:A76-A7.
- [PubMed] [Google Scholar]
- Treatment of chronic intractable urticaria in children with classical prescription: A case report. Shanxi Tradit Chin Med. 2019;35:45.
- [Google Scholar]
- Contact dermatitis and acute urticaria caused by Yunnan Baiyao wound patch. Strait Pharm J. 2020;32:219-20.
- [Google Scholar]
- Kimura disease complicated with chronic urticaria: a case report. Chin J Dermatol. 2022;55:812-3.
- [Google Scholar]
- Ketotifen combined with cetirizine hydrochloride in the treatment of 34 cases of chronic urticaria. Prac Clin Med. 2007;8:38.
- [Google Scholar]
- Observation on the efficacy and quality of life of levocetirizine combined with ketotifen in the treatment of chronic idiopathic urticaria. Shandong Med J. 2008;48:97-8.
- [Google Scholar]
- Clinical observation of cyproheptadine combined with cetirizine in the treatment of chronic urticaria. Med Front. 2011;1:58-9.
- [Google Scholar]
- Therapeutic effect of Xiaochaihu Decoction plus levocetirizine on acute urticaria and its effect on TLR4 and TLR2 levels of monocytes in patients with acute urticaria. J Chin Prescript Drug. 2020;18:131-3.
- [Google Scholar]
- Influence of Jin’s three -needle combined with desloratadine in treating of acute urticaria on effect and peripheral blood mononuclear cells TLR4 and TLR2 levels. Chin J Dermatovenerol Integr Trad Western Med. 2018;17:200-3.
- [Google Scholar]
- Effect and laboratory data analysis of levocetirizine hydrochloride combined with total glucosides of paeony in treatment of acute urticaria. Smart Healthcare. 2017;3:80-2.
- [Google Scholar]
- Clinical observation of Xiyanping combined with desloratadine in the treatment of acute urticaria. Med Front 2013:160-1.
- [Google Scholar]
- Ebastine in the treatment of allergic rhinitis and urticaria: 30 years of clinical studies and real-world experience. J Investig Allergol Clin Immunol. 2020;30:156-68.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the efficacy and safety of doubling the dose of second-generation antihistamines in the treatment of chronic urticaria. Dermatol & Venereol. 2021;43:597-8.
- [PubMed] [Google Scholar]
- Cardiac safety of second-generation H1-antihistamines when updosed in chronic spontaneous urticaria. Clin Exp Allergy. 2019;49:1615-23.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of up-dosing antihistamines in chronic spontaneous urticaria: A systematic review of the literature. J Investig Allergol Clin Immunol. 2021;31:282-91.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of initial treatment modality on long-term control of chronic idiopathic urticaria. PLoS One. 2013;8:e69345.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of combination therapy on mortality and adverse events in patients with staphylococcus aureus bacteraemia: A systematic review and meta-analysis of randomized controlled trials. Infect Dis Ther. 2021;10:2643-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A review of antihistamines used during pregnancy. J Pharmacol Pharmacother. 2012;3:105-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






