Translate this page into:
Efficacy of salicylic acid peel in dermatophytosis
Corresponding author: Dr. Vikrant Saoji, 1st Floor, Midas Heights, Ramdaspeth, Nagpur, Maharashtra, India. vikrantsaoji@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol 2021;87:671-5.
Abstract
Background:
Treatment of dermatophytosis is becoming costlier and challenging.
Aims and Objectives:
To study the efficacy of salicylic acid peel in dermatophytosis.
Methods:
Twenty-five patients (20 males and 5 females) having dermatophytosis with positive potassium hydroxide (KOH) mounts were enrolled in the study. Salicylic acid 30% was applied over the lesions weekly for 4 weeks, thereafter patients were followed up weekly for 4 weeks.
Results:
Of the 25 patients, 22 (88%) patients showed clinical and microbiological cure 1 week after the last application, while the remaining 3 patients were nonresponders. Nine (41%) patients of the 22 responders showed recurrences indicating that 4 weeks’ treatment is not sufficient in some patients to eradicate fungus and may require longer treatment.
Limitations:
A relatively small sample size and lack of long-term follow-up are the shortcomings of our study.
Conclusion:
Salicylic acid peel is a cheap and useful option in the treatment of dermatophytic infection.
Keywords
Dermatophytosis
tinea
salicylic acid peel
topical treatment
Introduction
Although epidemiological data are lacking, it is a fact that there is an increase in the prevalence of dermatophytosis over the past few years across India.1,2 Standard treatment recommendations from western and Indian literature that we were following, seem no longer valid.1
Most antifungal drugs target ergosterol which is unique to the fungal cell wall and absent in mammalian cells. The disadvantage of targeting a single molecule is that an alteration in the target by natural selection can render antifungal drugs ineffective. In the absence of any new antifungal drug, treating dermatophytosis which was once considered a trivial infection has now become a problem of significant public health issue with financial implications. The treatment of multiple family members with higher doses and longer durations of antifungals has resulted in a significant financial burden for patients and their families.
Since the keratinophilic dermatophytes reside in the stratum corneum, peeling of this superficial layer should remove the fungus. We therefore studied 30% salicylic acid as a peeling agent for the treatment of dermatophytosis.
Materials and Methods
Patients suffering from active tinea infections with positive potassium hydroxide (KOH) mounts who were ready to participate in the study) were included. Pregnant females, children (younger than 18 years) and those with a negative KOH mount for fungus were excluded. Patients with extensive involvement (more than 20% body surface area) as well as those using topical or oral antifungals within the preceding 2 weeks were excluded. Itching was classified as per severity into three grades: mild itching - grade 1, moderate - grade 2, and severe - grade 3.
Salicylic acid 30% was prepared by adding acetone to 30 g of salicylic acid powder to make it 100 mL giving a 30% w/v salicylic acid. Salicylic acid 30% application was done over the lesions (and 1 cm beyond the lesional border). The maximum quantity of salicylic acid used during a single treatment session was 10 mL (3 g of salicylic acid). While applying salicylic acid in the inguinal area, care was taken to protect the scrotum. In case of severe burning, ice pack application was done.
The treatment was repeated every week (with a delay of up to 3 days considered acceptable) for 4 weeks. Thereafter, the patients were followed up weekly for four visits by the same set of investigators for all visits. .
No systemic or topical antifungal drugs were coprescribed during the study period. KOH mounts was done at the baseline visit for confirming the diagnosis and repeated at the end of fifth visit (1 week after the last application) for the assessment of the response, and then every week till 4 weeks after the last application.
Results
A total of 35 patients were recruited in our study. Five patients showed negative findings on KOH examination and five were left out of the study due to irregular adherence to study protocol. In all, 25 patients (20 males and 5 females) were included for analysis. Table 1 gives data on patients treated and the outcome of treatment. Patients’ age ranged from 20 to 58 years with a mean age of 33 years. Except two, all the patients had inguinal involvement. All the patients except one had more than one anatomical site involved [Table 1]. Three patients were treatment-naive, while the remaining 22 had received antifungal treatment in the past 6 months. There were seven patients with clinical resistance to oral terbinafine and one patient to oral itraconazole; the rest reported recurrences after initial improvement with antifungal treatment. A total of 22 (88%) patients had achieved clinical and microbiological clearance 1 week after the last salicylic acid application. Three patients were still KOH-positive at the end of the study period and also showed clinical activity; but these patients reported symptomatic improvement. The 7 patients with previous clinical resistance to terbinafine and the patient whose infection seemed resistant to itraconazole showed clinical and microbiological cure. Of 22 patients who achieved clearance with negative KOH findings, 9 (36% of study participants) developed recurrence (clinical and microbiological) during the follow-up period of 4 weeks. Of the seven patients with clinical resistance to terbinafine, four had recurrence. While on treatment, four patients reported new lesions at a distant site during second visit, which was treated with salicylic acid during the subsequent visit. There were three nonresponders, all with extensive disease and all three had previously used topical steroid and antifungal combinations. Ten patients gave a history of using over-the-counter topical steroid-antifungal combinations. Seventeen of 25 (68%) patients reported significant improvement (change of 2 grades) in itching after first application (visit 2). All non-responders experienced minimum improvement in itching. All patients reported a burning sensation during the application of salicylic acid over the inflamed area. One of the patients who had erosions in the affected area dropped out of the study as she experienced severe burning during the first salicylic acid application. Decreases in erythema and scaling were noticed during subsequent visits with improvement in the subjects who were able to complete the study. Patients reported decreased burning sensation during subsequent treatment sessions. None of the patients experienced any major (systemic) side effects due to salicylic acid. Figures 1-3 show the results of salicylic acid peel in our study patients.
| Age/sex | Area of involvement | Duration (months) | Prior treatment received | Outcome |
|---|---|---|---|---|
| 36/female | I/A | 5 | S, T (R to T) | CL |
| 21/male | I/B | 12 | F, T, G, S(R to T) | CL, Re after 4 weeks |
| 21/female | UL | 7 | S, T (R to T) | CL |
| 36/male | I, A | 24 | TCS + TAF, T(R to T) | CL |
| 28/female | I, LL | 6 | Nil | CL |
| 40/male | I, F | 8 | Nil | CL |
| 22/male | I, B | 5 | TCS + TAF, St-o | CL, Re after 2 weeks |
| 58/male | I, A | 3 | Nil | CL, Re after 4 weeks |
| 40/male | N, C, LL, F | 1 | TCS | CL |
| 29/male | I, B, LL | 6 | TAF | CL, Re after 4 weeks |
| 25/male | I, UL | 4 | TCS, TAF | CL, |
| 21/male | I, B | 3 | TCS + TAF | CL, Re after 1 week |
| 57/male | I, B, A, LL | 5 | TCS | CL, Re after 4 weeks |
| 26/male | I, B, A, C | 4 | T, I, K, TCS + TAF | Nonresponder |
| 38/female | I, B, LL | 2 | TCS + TAF | Nonresponder |
| 40/male | I, A, B | 3 | S, T | CL, Re after 1 week |
| 52/male | I, F, A, LL, UL, B | 4 | T, S(R to T) | CL, Re after 4 weeks |
| 23/female | I, LL, A | 6 | F, K | CL |
| 24/male | I, LL, B | 24 | I | CL |
| 37/male | B | 3 | CL | |
| 36/male | I, A, LL, UL | 5 | S, K | CL |
| 24/male | I, A, LL | 4 | TAF, TCS | CL |
| 28/male | I, F | 2 | TCS, TAF | CL |
| 27/male | I, LL, F | 4 | T (R to T) | CL, Re after 2 weeks |
| 24/male | I, B, UL | 2 | TCS | CL |
Area of involvement – B: buttock, C: chest, F: face, I: inguinal, LL: lower limb, UL: upper limb. Prior treatment received – F: fluconazole, G: griseofulvin, I: itraconazole, K: ketoconazole, R to T: clinical resistance to terbinafine, S: sertaconazole, St-o: oral steroids, TCS: topical corticosteroid, TAF: topical antifungal. Outcome – CL: clearance of lesions, Re: recurrence
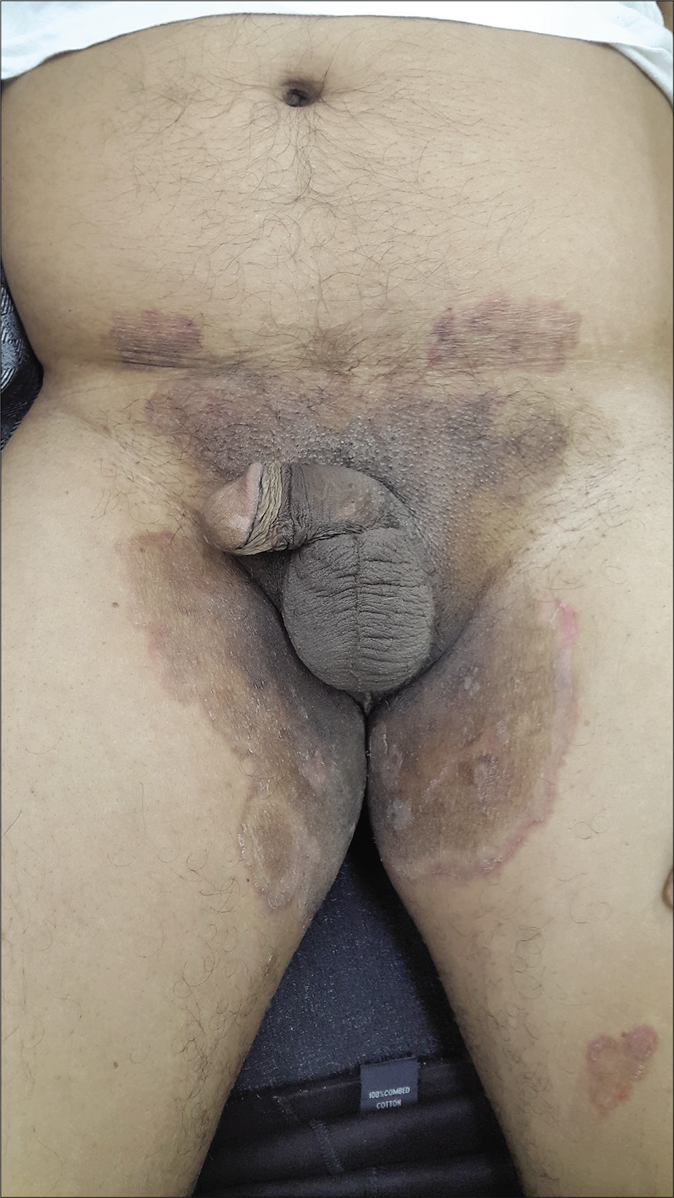
- Pre-treatment image of tinea cruris

- Post-treatment image of tinea cruris 4 weeks after treatment
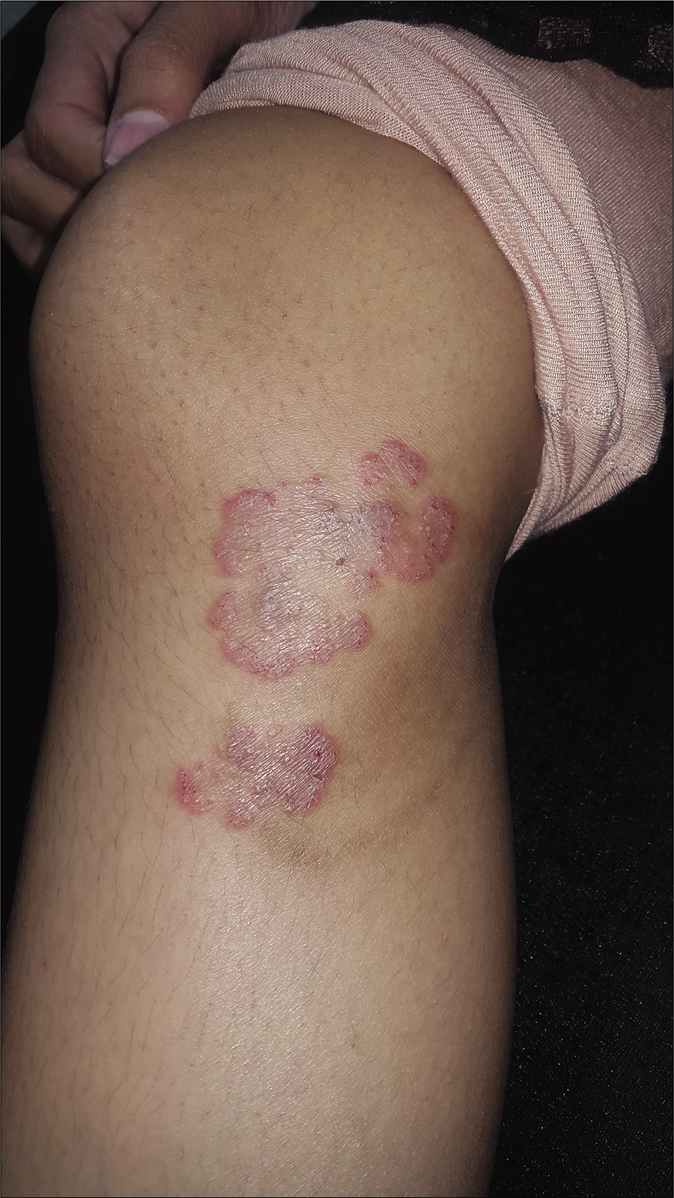
- Pre-treatment image of tinea corporis over knee
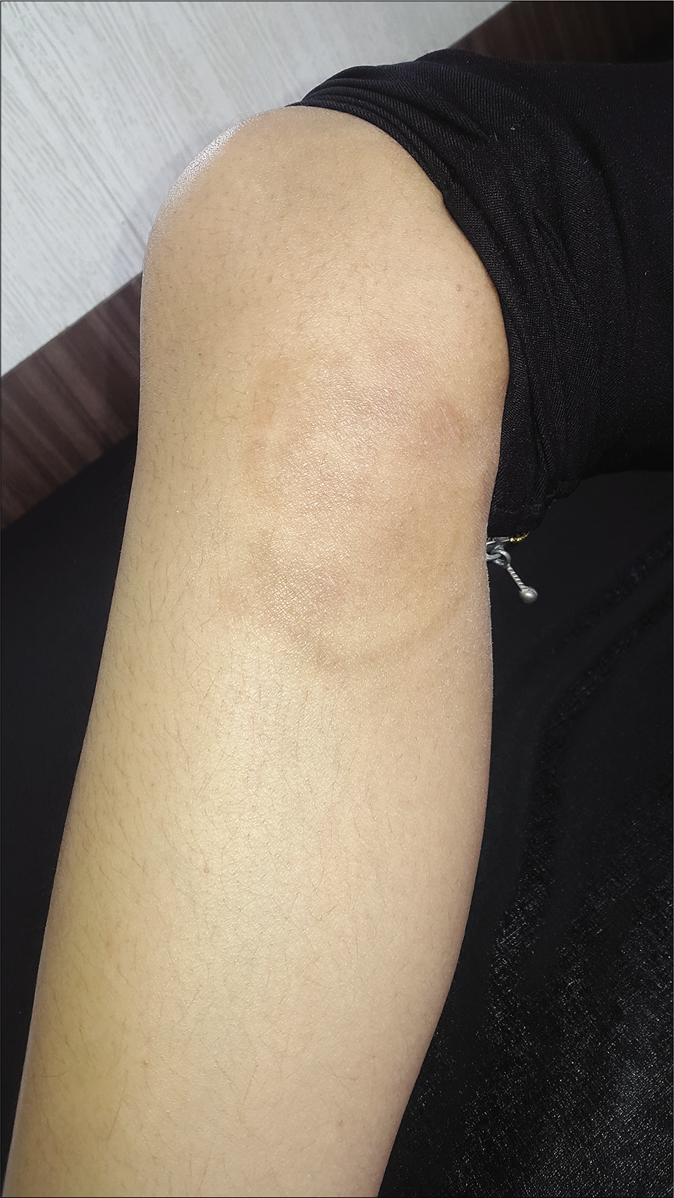
- Post-treatment image of tinea corporis after salicylic acid peeling (after one week of last application)
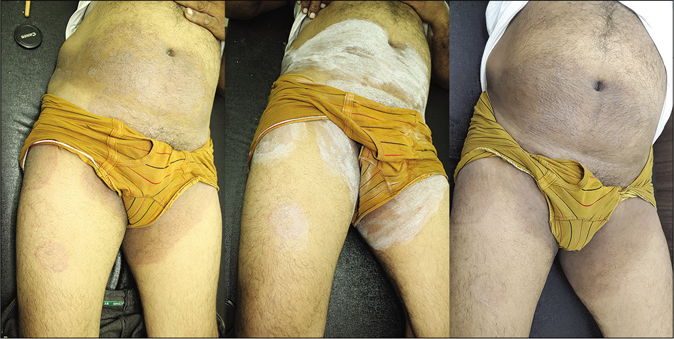
- Sequential photographs of a patient taken at baseline, immediately after salicylic acid application and one week after fourth application in a case of tinea cruris
Discussion
Salicylic acid (from Latin salix, willow tree) is a lipophilic monohydroxybenzoic acid, a type of phenolic acid, and a beta hydroxy acid. It is keratolytic and 20%–30% concentration is used as a peeling agent. 3% salicylic acid has been in use in Whitfield’s ointment for the treatment of superficial fungal infection especially tinea pedis. The Indian Association of Dermatologists, Venereologists Leprologists manual of dermatophytosis mentions 6% salicylic acid as an adjuvant in the treatment of dermatophytosis, to increase the penetration of topical antifungals.3
Salicylic acid can be stored at room temperature. Being a strong acid, it has a strong irritant potential and application near sensitive areas (eyes, nose, mouth, rectum, or vagina) should be avoided. Application of salicylic acid over the inflamed areas of tinea can cause a severe burning sensation and was noticed in all our patients.
Since dermatophytes remain in the stratum corneum, only superficial peels may be sufficient to remove them. However after achieving clearance, recurrence was noticed in several patients indicating that 4 weeks is not adequate to eradicate the fungus from the skin in all cases and longer treatment is perhaps required. Involvement of vellus hair follicles by the fungus may be responsible for recurrence, as these sites offer the protection to dermatophytes and may remain unreachable to the salicylic acid.4 Appearance of the lesions at the distant site indicates that topically applied salicylic acid has only a local action and no systemic effect.
Systemic toxicity due to cutaneous absorption of salicylic acid is a very rare phenomenon, but should be watched for. The clinical presentation of salicylic acid toxicity includes nausea, vomiting, dizziness, psychosis, stupor, and consequently coma and death.5 It should not be used in children below 2 years of age and during pregnancy. Salicylic acid is classified by the US Food and Drug Administration as a pregnancy category C drug. Although the protocol limit of maximum quantity of salicylic acid during any visit is 10 mL, most of our patients required 3–5 mL.
Taylor and Halprin found that 28 g of 6% salicylic acid when applied under occlusion for extensive psoriasis for 5 days does not lead to any significant serum level of salicylic acid and does not cause any adverse effect.6 Repeated salicylic acid 30% peeling on the face has also not resulted in any major side effects.7 There are no reported drug interactions between topical salicylic acid and other systemic antifungal agents, in a practical scenario; salicylic acid peeling can therefore be combined with systemic antifungal drugs for the management of tinea infection.
Topical steroid-antifungal combinations as prescribed by nondermatologists or purchased over-the-counter and used commonly by patients are probably an important factor in the current dermatophyte epidemic. Of the 10 patients who had used such combinations in our study, 3 turned out to be nonresponders and 2 patients developed recurrence.
Of seven patients with apparent clinical resistance to terbinafine, four patients developed recurrence. Of the nine patients who relapsed, four patients reported clinical resistance to terbinafine . Limitations of this study include the relatively limited number of patients and short period of follow- up. Fungal culture would have been a better option to study the efficacy of salicylic acid, but because of the low fungal culture positivity rate in our laboratory it was not attempted.
Since salicylic acid does not affect the fungus directly, it is not likely to induce resistance. Peeling of superficial skin using salicylic acid 30% was found to be safe and it may be a useful option for the treatment of resistant tinea infection especially in the absence of any new antifungal drugs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227-36.
- [Google Scholar]
- The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol Venereol Leprol. 2017;83:281-4.
- [CrossRef] [Google Scholar]
- Overview of causes and treatment of recalcitrant dermatophytoses In: Sardana K, ed. IADVL Manual on Management of Dermatophytoses (1st ed). India: CBS Publishers and Distributors Pvt. Ltd.; 2018. p. :80-104.
- [Google Scholar]
- Tinea of vellus hair: An indication for systemic antifungal therapy. Br J Dermatol. 2010;163:603-6.
- [CrossRef] [Google Scholar]
- Salicylic acid as a peeling agent: A comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-61.
- [CrossRef] [Google Scholar]
- Percutaneous absorption of salicylic acid. Arch Dermatol. 1975;111:740-3.
- [CrossRef] [Google Scholar]
- Tolerance and safety of superficial chemical peeling with salicylic acid in various facial dermatoses. Indian J Dermatol Venereol Leprol. 2005;71:87-90.
- [CrossRef] [Google Scholar]






